当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 7'-Arylidenespiro[indoline-3,1'-pyrrolizines] and 7'-Arylidenespiro[indene-2,1'-pyrrolizines] via [3 + 2] Cycloaddition and β-C-H Functionalized Pyrrolidine.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-09-19 , DOI: 10.1021/acs.joc.9b01920 Ying Huang 1, 2 , Hui-Lin Fang 1 , Yi-Xin Huang 2 , Jing Sun 1 , Chao-Guo Yan 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-09-19 , DOI: 10.1021/acs.joc.9b01920 Ying Huang 1, 2 , Hui-Lin Fang 1 , Yi-Xin Huang 2 , Jing Sun 1 , Chao-Guo Yan 1
Affiliation
|
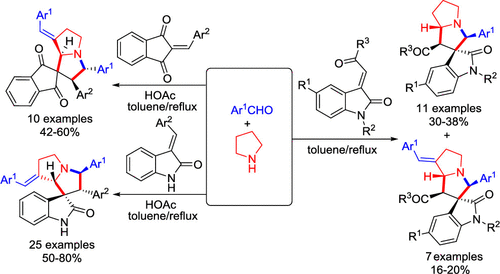
The acetic acid catalyzed three-component reaction of pyrrolidine, aromatic aldehydes, and 3-arylideneoxindolin-2-ones in refluxing toluene afforded functionalized 7'-arylidenespiro[indoline-3,1'-pyrrolizines] in good yields and with high diastereoselectivity. The similar three-component reaction with 2-arylidene-1,3-indanediones also gave 7'-arylidenespiro[indene-2,1'-pyrrolizines] in good yields. However, the reaction with 3-phenacylideneoxindoles resulted in a mixture of spiro[indoline-3,1'-pyrrolizines] and 7'-arylidene-substituted spirooxindoles in moderate yields. The reaction mechanism included generation of azomethine ylides, β-C-H functionalization of pyrrolidine, and sequential [3 + 2] cycloaddition. The obtained spirooxindole derivatives were investigated by in vitro evaluation against mouse colon cancer cells CT26 and human liver cancer cells HepG2 by MTT assay.
中文翻译:

通过[3 + 2]环加成反应和β-CH官能化吡咯烷合成7'-Arylidenespiro [吲哚啉-3,1'-吡咯嗪]和7'-Arylidenespiro [indene-2,1'-吡咯嗪]。
乙酸在回流的甲苯中催化吡咯烷,芳族醛和3-芳基氧杂吲哚-2-酮的三组分反应,可提供高收率和高非对映选择性的功能化7'-芳基螺[吲哚啉-3,1'-吡咯烷酮]。与2-亚芳基-1,3-茚满二酮的相似的三组分反应也以良好的产率得到了7'-亚芳基螺[茚-2,1'-吡咯烷酮]。然而,与3-苯乙叉基亚吲哚的反应以中等收率产生了螺[吲哚啉-3,1'-吡咯嗪]和7'-亚芳基取代的螺氧并吲哚的混合物。反应机理包括偶氮甲亚胺的生成,吡咯烷的β-CH官能化以及连续的[3 + 2]环加成反应。
更新日期:2019-09-19
中文翻译:

通过[3 + 2]环加成反应和β-CH官能化吡咯烷合成7'-Arylidenespiro [吲哚啉-3,1'-吡咯嗪]和7'-Arylidenespiro [indene-2,1'-吡咯嗪]。
乙酸在回流的甲苯中催化吡咯烷,芳族醛和3-芳基氧杂吲哚-2-酮的三组分反应,可提供高收率和高非对映选择性的功能化7'-芳基螺[吲哚啉-3,1'-吡咯烷酮]。与2-亚芳基-1,3-茚满二酮的相似的三组分反应也以良好的产率得到了7'-亚芳基螺[茚-2,1'-吡咯烷酮]。然而,与3-苯乙叉基亚吲哚的反应以中等收率产生了螺[吲哚啉-3,1'-吡咯嗪]和7'-亚芳基取代的螺氧并吲哚的混合物。反应机理包括偶氮甲亚胺的生成,吡咯烷的β-CH官能化以及连续的[3 + 2]环加成反应。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号