当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Water on CO2 Capture by Aprotic Heterocyclic Anion (AHA) Ionic Liquids
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-09-20 , DOI: 10.1021/acssuschemeng.9b04424 Gabriela M. Avelar Bonilla 1 , Oscar Morales-Collazo 1 , Joan F. Brennecke 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-09-20 , DOI: 10.1021/acssuschemeng.9b04424 Gabriela M. Avelar Bonilla 1 , Oscar Morales-Collazo 1 , Joan F. Brennecke 1
Affiliation
|
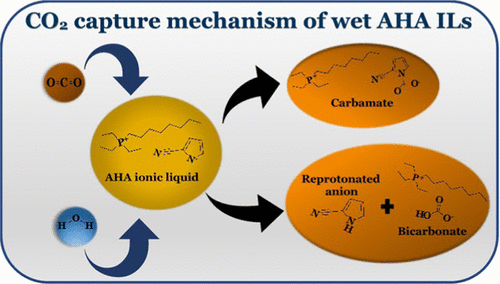
The reaction between three aprotic heterocyclic anion (AHA) ionic liquids (ILs) (triethyl(octyl)phosphonium 2-cyanopyrrolide [P2228][2CNPyr], triethylphosphonium benzimidazolide [P2222][BnIm], and triethyl(octyl)phosphonium indazolide [P2228][Inda]) and CO2, in the presence of water, is investigated in this study. We propose a reaction mechanism where, in addition to the reaction of the anion with CO2 to form carbamate, the anion reacts with water (i.e., hydronium ions in water from either the natural dissociation of water or the formation of carbonic acid) and is reprotonated, leaving CO2 to react with hydroxide to form bicarbonate. This mechanism is confirmed for [P2228][2CNPyr] and [P2222][BnIm] by 1H NMR, 13C NMR, heteronuclear single quantum correlation NMR, and heteronuclear multiple bond correlation NMR, which were used to identify and quantify the reaction products. However, unlike the other two ILs, [P2228][Inda] exhibits reprotonation of the anion when in contact with water only; i.e., CO2 is not needed to form carbonic acid to increase the proton concentration in water. The reversibility of the reprotonation reaction was tested by measuring the CO2 solubility in [P2228][2CNPyr] and [P2222][BnIm] in the presence of water for several cycles using a volumetric method. Previously, we have shown that the reaction of AHA ILs with CO2 to form carbamate (in the absence of water) is reversible. Additionally, encapsulated [P2228][2CNPyr] and [P2222][BnIm] were subjected to a CO2 solubility recyclability test in the presence of water. Both reactions between AHA ILs and CO2 in the presence of water appear to be fully reversible. The encapsulated ILs showed good CO2 capture capacity in the presence of water and good recyclability results, as well.
中文翻译:

水对非质子性杂环阴离子(AHA)离子液体捕获CO 2的影响
三种质子惰性杂环离子(AHA)离子液体(ILs)(三乙基(辛基)2- 2-氰基吡咯[P 2228 ] [2CNPyr],三乙基phosph苯并咪唑啉[P 2222 ] [BnIm]和三乙基(辛基))吲哚并[在这项研究中研究了在水存在下P 2228 ] [Inda]和CO 2的存在。我们提出了一种反应机理,其中,除了阴离子与CO 2的反应生成氨基甲酸酯以外,阴离子还与水反应(即,水的自然离解或碳酸形成的水中的水合氢根离子),并且再质子化,留下CO 2与氢氧化物反应形成碳酸氢盐。对于[P通过1 H NMR,13 C NMR,异核单量子相关NMR和异核多键相关NMR 2228 [2CNPyr]和[P 2222 ] [BnIm]鉴定和定量反应产物。然而,与其他两个IL不同,[P 2228 ] Inda仅在与水接触时表现出阴离子的质子化。也就是说,不需要CO 2形成碳酸来增加水中的质子浓度。通过测量[P 2228 ] [2CNPyr]和[P 2222 ]中CO 2的溶解度来测试质子化反应的可逆性在水的存在下使用体积法测定] [BnIm]。以前,我们已经证明AHA IL与CO 2形成氨基甲酸酯(在不存在水的情况下)的反应是可逆的。另外,将封装的[P 2228 ] [2CNPyr]和[P 2222 ] [BnIm]在水的存在下进行CO 2溶解度可循环性测试。在水的存在下,AHA IL和CO 2之间的两个反应似乎都是完全可逆的。包封的IL在水的存在下显示出良好的CO 2捕获能力,并具有良好的可回收性结果。
更新日期:2019-09-21
中文翻译:

水对非质子性杂环阴离子(AHA)离子液体捕获CO 2的影响
三种质子惰性杂环离子(AHA)离子液体(ILs)(三乙基(辛基)2- 2-氰基吡咯[P 2228 ] [2CNPyr],三乙基phosph苯并咪唑啉[P 2222 ] [BnIm]和三乙基(辛基))吲哚并[在这项研究中研究了在水存在下P 2228 ] [Inda]和CO 2的存在。我们提出了一种反应机理,其中,除了阴离子与CO 2的反应生成氨基甲酸酯以外,阴离子还与水反应(即,水的自然离解或碳酸形成的水中的水合氢根离子),并且再质子化,留下CO 2与氢氧化物反应形成碳酸氢盐。对于[P通过1 H NMR,13 C NMR,异核单量子相关NMR和异核多键相关NMR 2228 [2CNPyr]和[P 2222 ] [BnIm]鉴定和定量反应产物。然而,与其他两个IL不同,[P 2228 ] Inda仅在与水接触时表现出阴离子的质子化。也就是说,不需要CO 2形成碳酸来增加水中的质子浓度。通过测量[P 2228 ] [2CNPyr]和[P 2222 ]中CO 2的溶解度来测试质子化反应的可逆性在水的存在下使用体积法测定] [BnIm]。以前,我们已经证明AHA IL与CO 2形成氨基甲酸酯(在不存在水的情况下)的反应是可逆的。另外,将封装的[P 2228 ] [2CNPyr]和[P 2222 ] [BnIm]在水的存在下进行CO 2溶解度可循环性测试。在水的存在下,AHA IL和CO 2之间的两个反应似乎都是完全可逆的。包封的IL在水的存在下显示出良好的CO 2捕获能力,并具有良好的可回收性结果。





























 京公网安备 11010802027423号
京公网安备 11010802027423号