Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of NaV channels in synaptic transmission after axotomy in a microfluidic culture platform.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-09 , DOI: 10.1038/s41598-019-49214-w
Nickolai Vysokov 1 , Stephen B McMahon 1 , Ramin Raouf 1
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-09 , DOI: 10.1038/s41598-019-49214-w
Nickolai Vysokov 1 , Stephen B McMahon 1 , Ramin Raouf 1
Affiliation
|
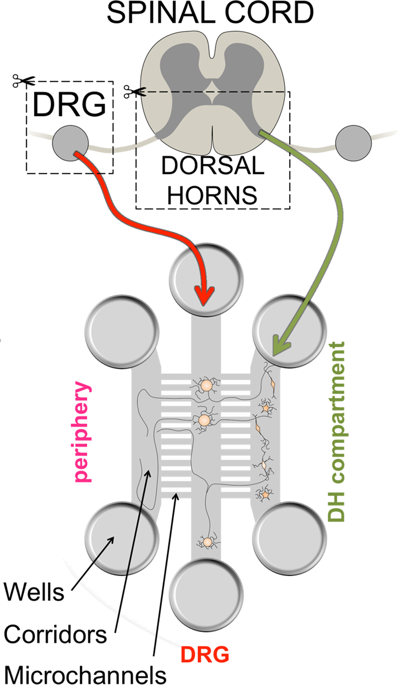
Voltage gated sodium channels are key players in aberrant pain signaling and sensitization of nociceptors after peripheral nerve injury. The extent to which sodium channel activity after injury contributes to synaptic transmission at the first pain synapse however remains unclear. To investigate the effect of axotomy on synaptic transmission between dorsal root ganglia neurons and dorsal horn neurons, we reconstructed the first pain synapse in a novel microfluidic based compartmentalized cell culture system, which recapitulates the connectivity of peripheral pain signaling. We show that following axotomy of the distal axons, inhibition of NaV1.7 and NaV1.8 sodium channels in incoming presynaptic DRG axons is no longer sufficient to block activation of these axons and the resulting synaptic transmission to dorsal horn neurons. We found that blockade of NaV1.6 activity is highly effective in reducing activation of incoming axons contributing to synaptic transmission after axotomy of DRG neurons. The microfluidic culture system described here offers an in vitro platform to recapitulate and study the first pain synapse.
中文翻译:

NaV通道在微流控培养平台中进行轴突切开后在突触传递中的作用。
电压门控钠通道是周围神经损伤后异常疼痛信号传导和伤害感受器敏化的关键因素。损伤后钠通道活性在第一次疼痛突触中对突触传递的贡献程度尚不清楚。为了研究轴突切开术对背根神经节神经元和背角神经元之间的突触传递的影响,我们在基于微流的新型隔室细胞培养系统中重建了第一个疼痛突触,概括了周围疼痛信号的连通性。我们显示,在对远端轴突进行轴突切开之后,抑制传入突触前DRG轴突中NaV1.7和NaV1.8钠通道的作用已不足以阻止这些轴突的激活以及由此产生的突触传递到背角神经元。我们发现,NaV1.6活性的阻断在减少DRG神经元轴突切开后有助于突触传递的传入轴突的激活方面非常有效。这里描述的微流控培养系统提供了一个体外平台来概括和研究第一个疼痛突触。
更新日期:2019-09-09
中文翻译:

NaV通道在微流控培养平台中进行轴突切开后在突触传递中的作用。
电压门控钠通道是周围神经损伤后异常疼痛信号传导和伤害感受器敏化的关键因素。损伤后钠通道活性在第一次疼痛突触中对突触传递的贡献程度尚不清楚。为了研究轴突切开术对背根神经节神经元和背角神经元之间的突触传递的影响,我们在基于微流的新型隔室细胞培养系统中重建了第一个疼痛突触,概括了周围疼痛信号的连通性。我们显示,在对远端轴突进行轴突切开之后,抑制传入突触前DRG轴突中NaV1.7和NaV1.8钠通道的作用已不足以阻止这些轴突的激活以及由此产生的突触传递到背角神经元。我们发现,NaV1.6活性的阻断在减少DRG神经元轴突切开后有助于突触传递的传入轴突的激活方面非常有效。这里描述的微流控培养系统提供了一个体外平台来概括和研究第一个疼痛突触。







































 京公网安备 11010802027423号
京公网安备 11010802027423号