当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibrium Study of Binary Mixtures of Chloroform, 2-Ethylhexanoic Acid, and Propylene Glycol Methyl Ether at Atmospheric Pressure
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-09-09 , DOI: 10.1021/acs.jced.9b00673 Yixin Ma 1 , Jun Gao 1 , Dongmei Xu 1 , Lianzheng Zhang 1 , Yinglong Wang
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-09-09 , DOI: 10.1021/acs.jced.9b00673 Yixin Ma 1 , Jun Gao 1 , Dongmei Xu 1 , Lianzheng Zhang 1 , Yinglong Wang
Affiliation
|
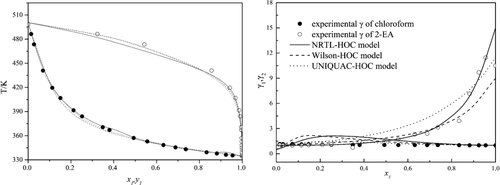
To separate the minimum-boiling azeotrope (propylene glycol methyl ether + water) in the production process of propylene glycol methyl ether, two extractive agents of chloroform and 2-ethylhexanoic acid were selected. The isobaric vapor–liquid phase equilibrium (VLE) data for three systems (chloroform + 2-ethylhexanoic acid), (propylene glycol methyl ether + 2-ethylhexanoic acid), and (chloroform + propylene glycol methyl ether) were measured at 101.3 kPa. Thermodynamic consistency of the measured VLE data was validated by the Herington and van Ness tests. The measured VLE data were well fitted with Hayden-O’Connell (HOC) thermodynamic models: nonrandom two-liquid-HOC, Wilson-HOC, and universal quasichemical-HOC with regressed parameters, respectively.
中文翻译:

大气压下氯仿,2-乙基己酸和丙二醇甲醚的二元混合物的汽液平衡研究
为了在丙二醇甲醚的生产过程中分离出最低沸点的共沸物(丙二醇甲醚+水),选择了氯仿和2-乙基己酸的两种萃取剂。在101.3 kPa下测量了三个系统(氯仿+ 2-乙基己酸),(丙二醇甲基醚+ 2-乙基己酸)和(氯仿+丙二醇甲基醚)的等压蒸气-液相平衡(VLE)数据。通过Herington和van Ness测试验证了所测VLE数据的热力学一致性。所测得的VLE数据与Hayden-O'Connell(HOC)热力学模型完全吻合:分别具有回归参数的非随机两液HOC,Wilson HOC和通用拟化学HOC。
更新日期:2019-09-09
中文翻译:

大气压下氯仿,2-乙基己酸和丙二醇甲醚的二元混合物的汽液平衡研究
为了在丙二醇甲醚的生产过程中分离出最低沸点的共沸物(丙二醇甲醚+水),选择了氯仿和2-乙基己酸的两种萃取剂。在101.3 kPa下测量了三个系统(氯仿+ 2-乙基己酸),(丙二醇甲基醚+ 2-乙基己酸)和(氯仿+丙二醇甲基醚)的等压蒸气-液相平衡(VLE)数据。通过Herington和van Ness测试验证了所测VLE数据的热力学一致性。所测得的VLE数据与Hayden-O'Connell(HOC)热力学模型完全吻合:分别具有回归参数的非随机两液HOC,Wilson HOC和通用拟化学HOC。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号