Nature Catalysis ( IF 42.8 ) Pub Date : 2019-09-09 , DOI: 10.1038/s41929-019-0336-1 Weilong Lin 1 , Ke-Feng Zhang 1 , Olivier Baudoin 1
|
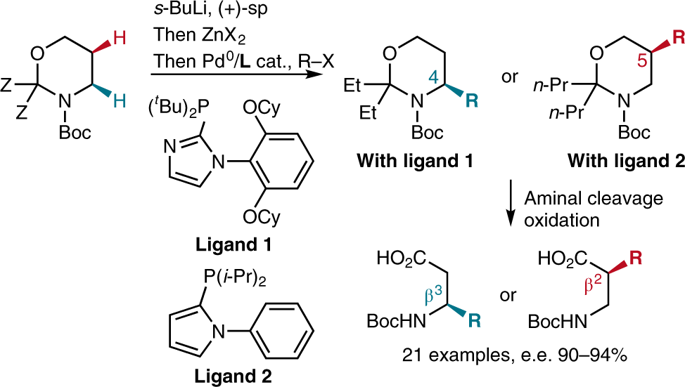
β2- and β3-amino acids are important chiral building blocks for the design of new pharmaceuticals and peptidomimetics. Here, we report a straightforward regio- and enantiodivergent access to these compounds using a one-pot reaction composed of sparteine-mediated enantioselective lithiation of a Boc-1,3-oxazinane, transmetallation to zinc and direct or migratory Negishi coupling with an organic electrophile. The regioselectivity of the Negishi coupling was highly ligand-controlled and switchable to obtain the C4- or the C5-functionalized product exclusively. High enantioselectivities were achieved on a broad range of examples, and a catalytic version in chiral diamine was developed using the (+)-sparteine surrogate. Selected C4- and C5-functionalized Boc-1,3-oxazinanes were subsequently converted to highly enantioenriched β2- and β3-amino acids with the (R) or (S) configuration, depending on the sparteine enantiomer employed in the lithiation step.
中文翻译:

Boc-1,3-恶嗪烷的区域发散对映选择性 C-H 官能化合成 β 2 - 和 β 3 - 氨基酸
β 2 - 和 β 3-氨基酸是设计新药物和肽模拟物的重要手性构件。在这里,我们报告了使用由sparteine 介导的Boc-1,3-oxazinane 的对映选择性锂化、金属转移到锌以及与有机亲电试剂直接或迁移的Negishi 偶联组成的单锅反应,直接获得这些化合物的区域和对映发散性。 . Negishi 偶联的区域选择性是高度配体控制的,并且可切换以专门获得 C4 或 C5 功能化产物。在广泛的示例中实现了高对映选择性,并且使用 (+)-sparteine 替代物开发了手性二胺中的催化形式。选定的 C4 和 C5 官能化 Boc-1,3-oxazinanes 随后转化为高度对映体富集的β2- 和 β 3 - 具有 ( R ) 或 ( S ) 构型的氨基酸,取决于锂化步骤中使用的斯巴丁对映异构体。































 京公网安备 11010802027423号
京公网安备 11010802027423号