当前位置:
X-MOL 学术
›
Acta Pharm. Sin. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cdk5 knocking out mediated by CRISPR-Cas9 genome editing for PD-L1 attenuation and enhanced antitumor immunity.
Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2019-07-23 , DOI: 10.1016/j.apsb.2019.07.004
Huan Deng 1 , Songwei Tan 1 , Xueqin Gao 1 , Chenming Zou 1 , Chenfeng Xu 1 , Kun Tu 1 , Qingle Song 1 , Fengjuan Fan 2 , Wei Huang 3 , Zhiping Zhang 1, 4, 5
Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2019-07-23 , DOI: 10.1016/j.apsb.2019.07.004
Huan Deng 1 , Songwei Tan 1 , Xueqin Gao 1 , Chenming Zou 1 , Chenfeng Xu 1 , Kun Tu 1 , Qingle Song 1 , Fengjuan Fan 2 , Wei Huang 3 , Zhiping Zhang 1, 4, 5
Affiliation
|
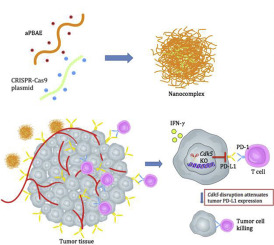
Blocking the programmed death-ligand 1 (PD-L1) on tumor cells with monoclonal antibody therapy has emerged as powerful weapon in cancer immunotherapy. However, only a minority of patients presented immune responses in clinical trials. To develop an alternative treatment method based on immune checkpoint blockade, we designed a novel and efficient CRISPR-Cas9 genome editing system delivered by cationic copolymer aPBAE to downregulate PD-L1 expression on tumor cells via specifically knocking out Cyclin-dependent kinase 5 (Cdk5) gene in vivo. The expression of PD-L1 on tumor cells was significantly attenuated by knocking out Cdk5, leading to effective tumor growth inhibition in murine melanoma and lung metastasis suppression in triple-negative breast cancer. Importantly, we demonstrated that aPBAE/Cas9-Cdk5 treatment elicited strong T cell-mediated immune responses in tumor microenvironment that the population of CD8+ T cells was significantly increased while regulatory T cells (Tregs) was decreased. It may be the first case to exhibit direct in vivo PD-L1 downregulation via CRISPR-Cas9 genome editing technology for cancer therapy. It will provide promising strategy for preclinical antitumor treatment through the combination of nanotechnology and genome engineering.
中文翻译:

通过 CRISPR-Cas9 基因组编辑介导的 Cdk5 敲除可减弱 PD-L1 并增强抗肿瘤免疫力。
利用单克隆抗体疗法阻断肿瘤细胞上的程序性死亡配体 1 (PD-L1) 已成为癌症免疫疗法的有力武器。然而,在临床试验中,只有少数患者出现免疫反应。为了开发基于免疫检查点阻断的替代治疗方法,我们设计了一种新型高效的 CRISPR-Cas9 基因组编辑系统,该系统由阳离子共聚物 aPBAE 提供,通过特异性敲除细胞周期蛋白依赖性激酶 5 (Cdk5) 来下调肿瘤细胞上的 PD-L1 表达体内的基因。通过敲除Cdk5,肿瘤细胞上PD-L1的表达显着减弱,从而有效抑制小鼠黑色素瘤的肿瘤生长和三阴性乳腺癌的肺转移。重要的是,我们证明 aPBAE/Cas9-Cdk5 治疗在肿瘤微环境中引发强烈的 T 细胞介导的免疫反应,CD8+ T 细胞数量显着增加,而调节性 T 细胞 (Treg) 减少。这可能是第一个通过 CRISPR-Cas9 基因组编辑技术在体内直接下调 PD-L1 用于癌症治疗的病例。它将通过纳米技术和基因组工程的结合为临床前抗肿瘤治疗提供有前景的策略。
更新日期:2019-09-09
中文翻译:

通过 CRISPR-Cas9 基因组编辑介导的 Cdk5 敲除可减弱 PD-L1 并增强抗肿瘤免疫力。
利用单克隆抗体疗法阻断肿瘤细胞上的程序性死亡配体 1 (PD-L1) 已成为癌症免疫疗法的有力武器。然而,在临床试验中,只有少数患者出现免疫反应。为了开发基于免疫检查点阻断的替代治疗方法,我们设计了一种新型高效的 CRISPR-Cas9 基因组编辑系统,该系统由阳离子共聚物 aPBAE 提供,通过特异性敲除细胞周期蛋白依赖性激酶 5 (Cdk5) 来下调肿瘤细胞上的 PD-L1 表达体内的基因。通过敲除Cdk5,肿瘤细胞上PD-L1的表达显着减弱,从而有效抑制小鼠黑色素瘤的肿瘤生长和三阴性乳腺癌的肺转移。重要的是,我们证明 aPBAE/Cas9-Cdk5 治疗在肿瘤微环境中引发强烈的 T 细胞介导的免疫反应,CD8+ T 细胞数量显着增加,而调节性 T 细胞 (Treg) 减少。这可能是第一个通过 CRISPR-Cas9 基因组编辑技术在体内直接下调 PD-L1 用于癌症治疗的病例。它将通过纳米技术和基因组工程的结合为临床前抗肿瘤治疗提供有前景的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号