Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational evolution of the cofactor-binding site of cytochrome P450 reductase yields variants with increased activity towards specific cytochrome P450 enzymes.
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-07-26 , DOI: 10.1111/febs.14982 Silja J Strohmaier 1 , Weiliang Huang 1 , Jong-Min Baek 1 , Dominic J B Hunter 1 , Elizabeth M J Gillam 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-07-26 , DOI: 10.1111/febs.14982 Silja J Strohmaier 1 , Weiliang Huang 1 , Jong-Min Baek 1 , Dominic J B Hunter 1 , Elizabeth M J Gillam 1
Affiliation
|
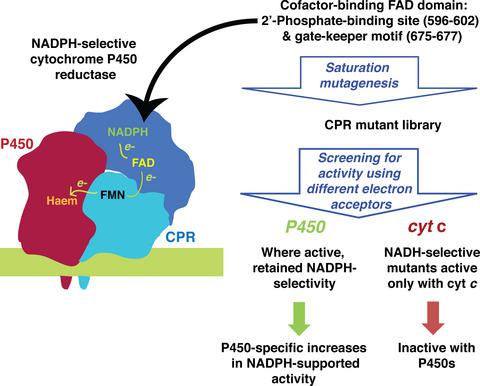
NADPH-cytochrome P450 reductase (CPR) is the natural redox partner of microsomal cytochrome P450 enzymes. CPR shows a stringent preference for NADPH over the less expensive cofactor, NADH, economically limiting its use as a biocatalyst. The complexity of cofactor-linked CPR protein dynamics and the incomplete understanding of the interaction of CPR with both cofactors and electron acceptors present challenges for the successful rational engineering of a CPR with enhanced activity with NADH. Here, we report a rational evolution approach to enhance the activity of CPR with NADH, in which mutations were introduced into the NADPH-binding flavin adenine dinucleotide (FAD) domain. Multiple CPR mutants that used NADH more effectively than the wild-type CPR in the reduction of the surrogate electron acceptor, cytochrome c were found. However, most were inactive in supporting P450 activity, arguing against the use of cytochrome c as a surrogate electron acceptor. Unexpectedly, several mutants showed significantly improved activity towards CYP2C9 (mutant 1-014) and/or CYP2A6 (mutants 1-014, 1-015, 1-053 and 1-077) using NADPH, even though the mutations were introduced at locations remote from the putative CPR-P450 interaction face. Therefore, mutations at sites in the FAD domain of CPR may be promising future engineering targets to enhance P450-mediated substrate turnover. ENZYMES: NADPH-cytochrome P450 reductase - EC 1.6.2.4; cytochrome P450 - EC 1.14.14.1.
中文翻译:

细胞色素P450还原酶辅因子结合位点的合理进化产生了对特定细胞色素P450酶具有增强活性的变体。
NADPH-细胞色素P450还原酶(CPR)是微粒体细胞色素P450酶的天然氧化还原伴侣。CPR对NADPH表现出比较便宜的辅因子NADH更为严格的要求,从经济上限制了其作为生物催化剂的用途。辅因子相关的CPR蛋白动力学的复杂性以及对CPR与辅因子和电子受体相互作用的不完全理解,为成功合理改造具有NADH活性的CPR提出了挑战。在这里,我们报告了一种合理的进化方法来增强NADH的CPR活性,其中将突变引入结合NADPH的黄素腺嘌呤二核苷酸(FAD)结构域。发现了多个CPR突变体,它们在替代电子受体细胞色素c的还原中比野生型CPR更有效地利用了NADH。然而,大多数人不支持P450活性,反对使用细胞色素c作为替代电子受体。出乎意料的是,即使将突变引入偏远地区,一些突变体仍显示使用NADPH对CYP2C9(突变体1-014)和/或CYP2A6(突变体1-014、1-015、1-053和1-077)具有显着改善的活性从推定的CPR-P450交互脸部获取。因此,CPR的FAD域中位点的突变可能是有希望的未来工程目标,以增强P450介导的底物更新。酵素:NADPH-细胞色素P450还原酶-EC 1.6.2.4; 细胞色素P450-EC 1.14.14.1。使用NADPH的几个突变体显示出对CYP2C9(突变体1-014)和/或CYP2A6(突变体1-014、1-015、1-053和1-077)的活性显着提高,即使该突变是在远离CYP2C9的位置引入的。推定的CPR-P450交互脸。因此,CPR的FAD域中位点的突变可能是有希望的未来工程目标,以增强P450介导的底物更新。酵素:NADPH-细胞色素P450还原酶-EC 1.6.2.4; 细胞色素P450-EC 1.14.14.1。使用NADPH的几个突变体显示出对CYP2C9(突变体1-014)和/或CYP2A6(突变体1-014、1-015、1-053和1-077)的活性显着提高,即使该突变是在远离CYP2C9的位置引入的。推定的CPR-P450交互脸。因此,CPR的FAD域中位点的突变可能是有希望的未来工程目标,以增强P450介导的底物更新。酵素:NADPH-细胞色素P450还原酶-EC 1.6.2.4; 细胞色素P450-EC 1.14.14.1。
更新日期:2019-07-26
中文翻译:

细胞色素P450还原酶辅因子结合位点的合理进化产生了对特定细胞色素P450酶具有增强活性的变体。
NADPH-细胞色素P450还原酶(CPR)是微粒体细胞色素P450酶的天然氧化还原伴侣。CPR对NADPH表现出比较便宜的辅因子NADH更为严格的要求,从经济上限制了其作为生物催化剂的用途。辅因子相关的CPR蛋白动力学的复杂性以及对CPR与辅因子和电子受体相互作用的不完全理解,为成功合理改造具有NADH活性的CPR提出了挑战。在这里,我们报告了一种合理的进化方法来增强NADH的CPR活性,其中将突变引入结合NADPH的黄素腺嘌呤二核苷酸(FAD)结构域。发现了多个CPR突变体,它们在替代电子受体细胞色素c的还原中比野生型CPR更有效地利用了NADH。然而,大多数人不支持P450活性,反对使用细胞色素c作为替代电子受体。出乎意料的是,即使将突变引入偏远地区,一些突变体仍显示使用NADPH对CYP2C9(突变体1-014)和/或CYP2A6(突变体1-014、1-015、1-053和1-077)具有显着改善的活性从推定的CPR-P450交互脸部获取。因此,CPR的FAD域中位点的突变可能是有希望的未来工程目标,以增强P450介导的底物更新。酵素:NADPH-细胞色素P450还原酶-EC 1.6.2.4; 细胞色素P450-EC 1.14.14.1。使用NADPH的几个突变体显示出对CYP2C9(突变体1-014)和/或CYP2A6(突变体1-014、1-015、1-053和1-077)的活性显着提高,即使该突变是在远离CYP2C9的位置引入的。推定的CPR-P450交互脸。因此,CPR的FAD域中位点的突变可能是有希望的未来工程目标,以增强P450介导的底物更新。酵素:NADPH-细胞色素P450还原酶-EC 1.6.2.4; 细胞色素P450-EC 1.14.14.1。使用NADPH的几个突变体显示出对CYP2C9(突变体1-014)和/或CYP2A6(突变体1-014、1-015、1-053和1-077)的活性显着提高,即使该突变是在远离CYP2C9的位置引入的。推定的CPR-P450交互脸。因此,CPR的FAD域中位点的突变可能是有希望的未来工程目标,以增强P450介导的底物更新。酵素:NADPH-细胞色素P450还原酶-EC 1.6.2.4; 细胞色素P450-EC 1.14.14.1。

































 京公网安备 11010802027423号
京公网安备 11010802027423号