Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New light on ancient enzymes - in vitro CO2 Fixation by Pyruvate Synthase of Desulfovibrio africanus and Sulfolobus acidocaldarius.
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-07-19 , DOI: 10.1111/febs.14981 Andreas Witt 1 , Roberta Pozzi 1 , Stephan Diesch 1 , Oliver Hädicke 2 , Hartmut Grammel 1
The FEBS Journal ( IF 5.5 ) Pub Date : 2019-07-19 , DOI: 10.1111/febs.14981 Andreas Witt 1 , Roberta Pozzi 1 , Stephan Diesch 1 , Oliver Hädicke 2 , Hartmut Grammel 1
Affiliation
|
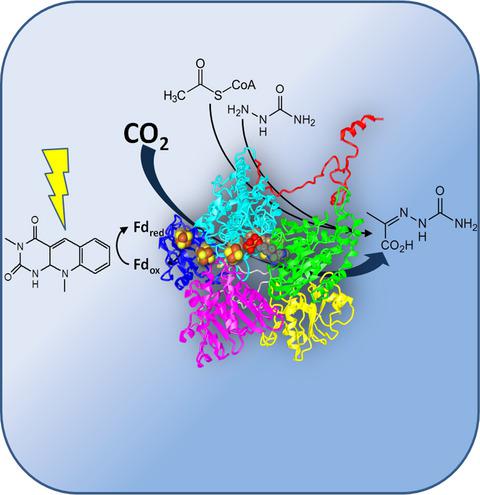
Two variants of the enzyme family pyruvate:ferredoxin oxidoreductase (PFOR), derived from the anaerobic sulfate-reducing bacterium Desulfovibrio africanus and the extremophilic crenarchaeon Sulfolobus acidocaldarius, respectively, were evaluated for their capacity to fixate CO2 in vitro. PFOR reversibly catalyzes the conversion of acetyl-CoA and CO2 to pyruvate using ferredoxin as redox partner. The oxidative decarboxylation of pyruvate is thermodynamically strongly favored, and most previous studies only considered the oxidative direction of the enzyme. To assay the pyruvate synthase function of PFOR during reductive carboxylation of acetyl-CoA is more challenging and requires to maintain the reaction far from equilibrium. For this purpose, a biochemical assay was established where low-potential electrons were introduced by photochemical reduction of EDTA/deazaflavin and the generated pyruvate was trapped by chemical derivatization with semicarbazide. The product of CO2 fixation could be detected as pyruvate semicarbazone by HPLC-MS. In a combinatorial approach, both PFORs were tested with ferredoxins from different sources. The pyruvate semicarbazone product could be detected with low-potential ferredoxins of the green sulfur bacterium Chlorobium tepidum and of S. acidocaldarius whereas CO2 fixation was not supported by the native ferredoxin of D. africanus. Methylviologen as an artificial electron carrier also allowed CO2 fixation. For both enzymes, the results are the first demonstration of CO2 fixation in vitro. Both enzymes exhibited high stability in the presence of oxygen during purification and storage. In conclusion, the employed PFOR enzymes in combination with non-native ferredoxin cofactors might be promising candidates for further incorporation in biocatalytic CO2 conversion. ENZYMES: EC1.2.7.1. Pyruvate:Ferredoxin Oxidoreductase.
中文翻译:

古代酶的新发现-非洲脱硫弧菌和嗜酸硫杆菌的丙酮酸合酶体外固定二氧化碳。
评估了丙酮酸酶家族的两个变体:铁氧还蛋白氧化还原酶(PFOR),它们分别来自减少厌氧硫酸盐的细菌Desulfovibrio africanus和极端嗜热的克雷纳氏嗜盐菌Sulfolobus acidocaldarius,在体外固定CO2的能力。使用铁氧还蛋白作为氧化还原伴侣,PFOR可逆地催化乙酰辅酶A和CO2转化为丙酮酸。丙酮酸的氧化脱羧在热力学上是强烈支持的,并且大多数先前的研究仅考虑了酶的氧化方向。要在乙酰辅酶A的还原羧化过程中测定PFOR的丙酮酸合酶功能更具挑战性,并且需要使反应远离平衡。以此目的,建立了一种生物化学测定法,其中通过光化学还原EDTA /脱氮黄素引入低电势电子,并通过用氨基脲进行化学衍生化来捕获生成的丙酮酸。HPLC-MS可以检测出二氧化碳固定的产物为丙酮酸氨基脲。通过组合方法,两个PFOR均用不同来源的铁氧还蛋白进行了测试。丙酮酸半卡巴zone产物可以用绿色硫磺细菌Chlorobium tepidum和S. acidocaldarius的低电位铁氧还蛋白检测到,而天然D. Africanus铁氧还蛋白不支持CO2固定。甲基紫罗兰作为人造电子载体也可以固定CO2。对于这两种酶,该结果均为体外CO2固定的首次证明。两种酶在纯化和储存过程中在氧气存在下均显示出高稳定性。总之,与非天然铁氧还蛋白辅因子结合使用的PFOR酶可能是有希望的候选物,可以进一步掺入生物催化CO2转化中。酶:EC1.2.7.1。丙酮酸:铁氧还蛋白氧化还原酶。
更新日期:2019-07-19
中文翻译:

古代酶的新发现-非洲脱硫弧菌和嗜酸硫杆菌的丙酮酸合酶体外固定二氧化碳。
评估了丙酮酸酶家族的两个变体:铁氧还蛋白氧化还原酶(PFOR),它们分别来自减少厌氧硫酸盐的细菌Desulfovibrio africanus和极端嗜热的克雷纳氏嗜盐菌Sulfolobus acidocaldarius,在体外固定CO2的能力。使用铁氧还蛋白作为氧化还原伴侣,PFOR可逆地催化乙酰辅酶A和CO2转化为丙酮酸。丙酮酸的氧化脱羧在热力学上是强烈支持的,并且大多数先前的研究仅考虑了酶的氧化方向。要在乙酰辅酶A的还原羧化过程中测定PFOR的丙酮酸合酶功能更具挑战性,并且需要使反应远离平衡。以此目的,建立了一种生物化学测定法,其中通过光化学还原EDTA /脱氮黄素引入低电势电子,并通过用氨基脲进行化学衍生化来捕获生成的丙酮酸。HPLC-MS可以检测出二氧化碳固定的产物为丙酮酸氨基脲。通过组合方法,两个PFOR均用不同来源的铁氧还蛋白进行了测试。丙酮酸半卡巴zone产物可以用绿色硫磺细菌Chlorobium tepidum和S. acidocaldarius的低电位铁氧还蛋白检测到,而天然D. Africanus铁氧还蛋白不支持CO2固定。甲基紫罗兰作为人造电子载体也可以固定CO2。对于这两种酶,该结果均为体外CO2固定的首次证明。两种酶在纯化和储存过程中在氧气存在下均显示出高稳定性。总之,与非天然铁氧还蛋白辅因子结合使用的PFOR酶可能是有希望的候选物,可以进一步掺入生物催化CO2转化中。酶:EC1.2.7.1。丙酮酸:铁氧还蛋白氧化还原酶。































 京公网安备 11010802027423号
京公网安备 11010802027423号