Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Reverse Micelles Formulated with the Ionic-Liquid-like Surfactant Bmim-AOT and Comparison with the Traditional Na-AOT: Dynamic Light Scattering, 1H NMR Spectroscopy, and Hydrolysis Reaction of Carbonate as a Probe.
Langmuir ( IF 3.7 ) Pub Date : 2019-09-19 , DOI: 10.1021/acs.langmuir.9b01083 Nahir Dib 1 , R Dario Falcone 1 , Angel Acuña 2 , Luis García-Río 2
Langmuir ( IF 3.7 ) Pub Date : 2019-09-19 , DOI: 10.1021/acs.langmuir.9b01083 Nahir Dib 1 , R Dario Falcone 1 , Angel Acuña 2 , Luis García-Río 2
Affiliation
|
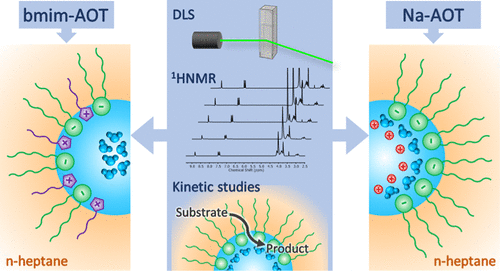
The present study investigated how the presence of butylmethylimidazolium cation (bmim+) alters the interfacial properties of reverse micelles (RMs) created with the ionic liquid-like surfactant 1-butyl-3-methylimidazolium 1,4-bis-2-ethylhexylsulfosuccinate (bmim-AOT), in comparison to sodium 1,4-bis-2-ethylhexylsulfosuccinate (Na-AOT) RMs, employing dynamic light scattering (DLS) and 1H NMR techniques. Moreover, through the hydrolysis reaction of bis(4-nitrophenyl)carbonate inside both RMs as reaction probe, interfacial properties changes were explored in more detail. The kinetic solvent isotope effect was also analyzed. Micellar systems were formed using n-heptane as external nonpolar solvent and water as the polar component. According to the DLS studies, water is encapsulated inside the organized media; however, a different tendency is observed depending on the cationic component of the surfactant. For Na-AOT system, the results suggest that the micellar shapes are probably spherical, while in the case of bmim-AOT, a transition from ellipsoidal to spherical micelles could be occurring when water is added. 1H NMR data show that water is structured differently when Na+ cation is replaced by bmim+; in bmim-AOT RMs, the interaction of water with the surfactant is weaker and the water hydrogen-bonding network is less disturbed than in Na-AOT RMs. Kinetic studies reveal that the hydrolysis reaction in bmim-AOT RMs was much more favorable in comparison to Na-AOT RMs. In addition, when water content decreases in bmim-AOT RMs, the hydrolysis reaction rate increases and the solvent isotope effect remains constant, while for Na-AOT solutions, both the reaction rate and the solvent isotope effect decrease. Our results indicate that bmim+ cation would be located in the surfactant layer in such a way the negative charge density in the interface is less than that in Na-AOT RMs, and the reaction is more favorable. Additionally, as 1H NMR studies reveal, the interfacial water molecules would be more available in bmim-AOT RMs to participate in the nucleophilic attack. Therefore, the present study evidences how the replacement of Na+ counterion by bmim+ alters the composition of the interface of AOT RMs.
中文翻译:

用离子液体样表面活性剂Bmim-AOT配制的反胶束的表征以及与传统Na-AOT的比较:动态光散射,1H NMR光谱和碳酸盐的水解反应作为探针。
本研究调查了丁基甲基咪唑阳离子(bmim +)的存在如何改变由离子液体状表面活性剂1-丁基-3-甲基咪唑1,4-双-2-乙基己基磺基琥珀酸酯(bmim-与1,4-双-2-乙基己基磺基琥珀酸钠(Na-AOT)RMs相比,采用动态光散射(DLS)和1H NMR技术。此外,通过在两个RM内作为反应探针的碳酸双(4-硝基苯基)碳酸酯的水解反应,更详细地研究了界面性质的变化。还分析了动力学溶剂的同位素效应。胶束系统是使用正庚烷作为外部非极性溶剂并以水作为极性成分形成的。根据DLS的研究,水被封装在有组织的介质中。然而,根据表面活性剂的阳离子组分,观察到不同的趋势。对于Na-AOT系统,结果表明胶束形状可能是球形,而在bmim-AOT的情况下,加水可能会发生从椭圆形到球形胶束的转变。1 H NMR数据表明,当用bmim +代替Na +阳离子时,水的结构不同。在bmim-AOT RMs中,水与表面活性剂的相互作用较Na-AOT RMs弱,并且水氢键网络受到的干扰较小。动力学研究表明,与Na-AOT RM相比,bmim-AOT RM中的水解反应更为有利。此外,当bmim-AOT RMs中的水含量降低时,水解反应速率增加,溶剂同位素效应保持恒定,而对于Na-AOT溶液,反应速率和溶剂同位素效应都会降低。我们的结果表明,bmim +阳离子将以这样一种方式位于表面活性剂层中:界面中的负电荷密度小于Na-AOT RMs中的负电荷密度,并且反应更加有利。此外,正如1 H NMR研究表明,界面水分子在bmim-AOT RM中将更易于参与亲核攻击。因此,本研究证明了bmim +替代Na +抗衡离子如何改变AOT RMs界面的组成。界面水分子在bmim-AOT RM中会更容易参与亲核攻击。因此,本研究证明了bmim +替代Na +抗衡离子如何改变AOT RMs界面的组成。界面水分子在bmim-AOT RM中会更容易参与亲核攻击。因此,本研究证明了bmim +替代Na +抗衡离子如何改变AOT RMs界面的组成。
更新日期:2019-09-19
中文翻译:

用离子液体样表面活性剂Bmim-AOT配制的反胶束的表征以及与传统Na-AOT的比较:动态光散射,1H NMR光谱和碳酸盐的水解反应作为探针。
本研究调查了丁基甲基咪唑阳离子(bmim +)的存在如何改变由离子液体状表面活性剂1-丁基-3-甲基咪唑1,4-双-2-乙基己基磺基琥珀酸酯(bmim-与1,4-双-2-乙基己基磺基琥珀酸钠(Na-AOT)RMs相比,采用动态光散射(DLS)和1H NMR技术。此外,通过在两个RM内作为反应探针的碳酸双(4-硝基苯基)碳酸酯的水解反应,更详细地研究了界面性质的变化。还分析了动力学溶剂的同位素效应。胶束系统是使用正庚烷作为外部非极性溶剂并以水作为极性成分形成的。根据DLS的研究,水被封装在有组织的介质中。然而,根据表面活性剂的阳离子组分,观察到不同的趋势。对于Na-AOT系统,结果表明胶束形状可能是球形,而在bmim-AOT的情况下,加水可能会发生从椭圆形到球形胶束的转变。1 H NMR数据表明,当用bmim +代替Na +阳离子时,水的结构不同。在bmim-AOT RMs中,水与表面活性剂的相互作用较Na-AOT RMs弱,并且水氢键网络受到的干扰较小。动力学研究表明,与Na-AOT RM相比,bmim-AOT RM中的水解反应更为有利。此外,当bmim-AOT RMs中的水含量降低时,水解反应速率增加,溶剂同位素效应保持恒定,而对于Na-AOT溶液,反应速率和溶剂同位素效应都会降低。我们的结果表明,bmim +阳离子将以这样一种方式位于表面活性剂层中:界面中的负电荷密度小于Na-AOT RMs中的负电荷密度,并且反应更加有利。此外,正如1 H NMR研究表明,界面水分子在bmim-AOT RM中将更易于参与亲核攻击。因此,本研究证明了bmim +替代Na +抗衡离子如何改变AOT RMs界面的组成。界面水分子在bmim-AOT RM中会更容易参与亲核攻击。因此,本研究证明了bmim +替代Na +抗衡离子如何改变AOT RMs界面的组成。界面水分子在bmim-AOT RM中会更容易参与亲核攻击。因此,本研究证明了bmim +替代Na +抗衡离子如何改变AOT RMs界面的组成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号