当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Runaway Reaction for the Esterification of Acetic Anhydride with Methanol Catalyzed by Sulfuric Acid
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acs.iecr.7b05160
Chiara Vianello 1 , Ernesto Salzano 2 , Alessio Broccanello 1 , Alessandro Manzardo 3 , Giuseppe Maschio 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2018-03-19 00:00:00 , DOI: 10.1021/acs.iecr.7b05160
Chiara Vianello 1 , Ernesto Salzano 2 , Alessio Broccanello 1 , Alessandro Manzardo 3 , Giuseppe Maschio 1
Affiliation
|
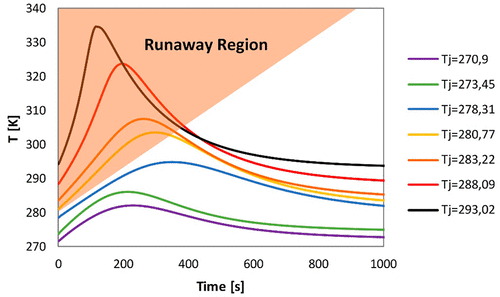
This work is devoted to the prevention of runaway reactions for the esterification of acetic anhydride with methanol in the presence of sulfuric acid. To this aim, a kinetic model has been developed and validated through the comparison with experimental data obtained by means of a batch reaction calorimeter operating in isoperibolic conditions. The model, which shows a good agreement with experimental data, then has been adopted for the prediction of the thermal runaway, which may lead to thermal explosion, through the definition of runaway criteria in a batch reactor and through the definition of stability diagram for the reactive system.
中文翻译:

硫酸催化甲醇催化乙酸酐酯化的失控反应
这项工作致力于防止在硫酸存在下乙酸酐与甲醇酯化的失控反应。为此,已经开发了动力学模型,并通过与在等代谢条件下运行的分批反应量热仪获得的实验数据进行比较来验证了该动力学模型。该模型显示出与实验数据的良好一致性,然后通过在间歇反应器中定义失控标准并通过定义反应堆的稳定性图,将其用于预测可能导致热爆炸的热失控。反应系统。
更新日期:2018-03-19
中文翻译:

硫酸催化甲醇催化乙酸酐酯化的失控反应
这项工作致力于防止在硫酸存在下乙酸酐与甲醇酯化的失控反应。为此,已经开发了动力学模型,并通过与在等代谢条件下运行的分批反应量热仪获得的实验数据进行比较来验证了该动力学模型。该模型显示出与实验数据的良好一致性,然后通过在间歇反应器中定义失控标准并通过定义反应堆的稳定性图,将其用于预测可能导致热爆炸的热失控。反应系统。



































 京公网安备 11010802027423号
京公网安备 11010802027423号