当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding Acidity of Molten Salt Hydrate Media for Cellulose Hydrolysis by Combining Kinetic Studies, Electrolyte Solution Modeling, Molecular Dynamics Simulations, and 13C NMR Experiments
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-23 , DOI: 10.1021/acscatal.9b03301 Natalia Rodriguez Quiroz 1 , Arul M. D. Padmanathan 2 , Samir H. Mushrif 2 , Dionisios G. Vlachos 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-23 , DOI: 10.1021/acscatal.9b03301 Natalia Rodriguez Quiroz 1 , Arul M. D. Padmanathan 2 , Samir H. Mushrif 2 , Dionisios G. Vlachos 1
Affiliation
|
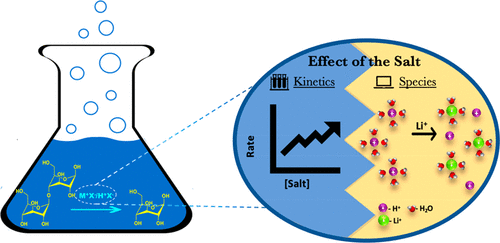
Depolymerization of lignocellulosic biomass in concentrated metal salts and more specifically in acidified LiBr molten salt hydrate (AMSH) results in high glucose yields at low acid concentrations, low temperatures, and very short times with potentially considerable economic benefits. However, our understanding of this promising medium is limited. Here, we study the effect of different LiBr concentrations on acidity and hydrolysis of cellobiose, a cellulose surrogate molecule, in dilute H2SO4 solutions. We use thermodynamic modeling to predict the H+(hydron) activity and the speciation and correlate these with the experimentally measured reaction rates. We find that the main contribution of the salt to the reactivity stems from the dramatic increase in H+ activity and secondary to an interaction of salt with the acid species that effectively renders the inorganic acid very strong. We perform molecular dynamics simulations and reveal that the increased hydron activity can be attributed to the decrease in the number of water molecules in the hydron solvation shell upon salt addition. Additionally, we extend the analysis to other salts and acids, concluding that the effects of different cations, anions, and acids in cellobiose hydrolysis likewise can be attributed to primarily changes in acidity. A key physicochemical descriptor of various salts is their enthalpy of dissolution. Finally, we explore the use of 13C NMR spectroscopy to estimate the pH of AMSH solutions.
中文翻译:

通过结合动力学研究,电解质溶液建模,分子动力学模拟和13 C NMR实验,了解用于纤维素水解的熔融盐水合物介质的酸度
木质纤维素生物质在浓金属盐中,特别是在酸化的LiBr熔融盐水合物(AMSH)中解聚,可在低酸浓度,低温和极短时间内获得高葡萄糖产量,具有潜在的显着经济效益。但是,我们对这种有前途的媒介的理解是有限的。在这里,我们研究了不同的LiBr浓度对酸度和纤维二糖的水解,纤维素替代分子,在稀H中的效果2 SO 4种溶液。我们使用热力学模型来预测H +(氢)活性和形态,并将其与实验测得的反应速率相关联。我们发现盐对反应性的主要贡献来自于H的急剧增加盐的+活性以及其次是盐与酸物质的相互作用,这使无机酸非常坚固。我们进行了分子动力学模拟,发现增加的水合活性可以归因于加盐后水合溶剂化壳中水分子数量的减少。此外,我们将分析扩展到其他盐和酸,得出结论,纤维二糖水解中不同阳离子,阴离子和酸的影响同样可以归因于酸度的主要变化。各种盐的关键理化指标是其溶解焓。最后,我们探索使用13 C NMR光谱估计AMSH溶液的pH。
更新日期:2019-10-24
中文翻译:

通过结合动力学研究,电解质溶液建模,分子动力学模拟和13 C NMR实验,了解用于纤维素水解的熔融盐水合物介质的酸度
木质纤维素生物质在浓金属盐中,特别是在酸化的LiBr熔融盐水合物(AMSH)中解聚,可在低酸浓度,低温和极短时间内获得高葡萄糖产量,具有潜在的显着经济效益。但是,我们对这种有前途的媒介的理解是有限的。在这里,我们研究了不同的LiBr浓度对酸度和纤维二糖的水解,纤维素替代分子,在稀H中的效果2 SO 4种溶液。我们使用热力学模型来预测H +(氢)活性和形态,并将其与实验测得的反应速率相关联。我们发现盐对反应性的主要贡献来自于H的急剧增加盐的+活性以及其次是盐与酸物质的相互作用,这使无机酸非常坚固。我们进行了分子动力学模拟,发现增加的水合活性可以归因于加盐后水合溶剂化壳中水分子数量的减少。此外,我们将分析扩展到其他盐和酸,得出结论,纤维二糖水解中不同阳离子,阴离子和酸的影响同样可以归因于酸度的主要变化。各种盐的关键理化指标是其溶解焓。最后,我们探索使用13 C NMR光谱估计AMSH溶液的pH。











































 京公网安备 11010802027423号
京公网安备 11010802027423号