当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Dynamics Simulations of an Initial Chemical Reaction Mechanism of Shocked CL-20 Crystals Containing Nanovoids
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-09-17 , DOI: 10.1021/acs.jpcc.9b06137 Fuping Wang 1 , Lang Chen 1 , Deshen Geng 1 , Jianying Lu 1 , Junying Wu 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-09-17 , DOI: 10.1021/acs.jpcc.9b06137 Fuping Wang 1 , Lang Chen 1 , Deshen Geng 1 , Jianying Lu 1 , Junying Wu 1
Affiliation
|
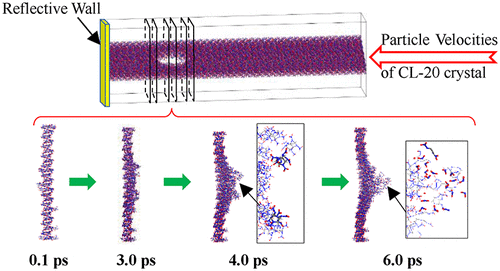
To understand the initial chemical reaction mechanism of the heterogeneous explosive hexanitrohexaazaisowurtzitane (CL-20), it is necessary to study the shock initiation mechanism of this nanovoid-containing crystal. In this paper, supercells of CL-20 with different void sizes were constructed. The chemical reactions induced by different impact velocities were calculated using molecular dynamics based on the ReaxFF-lg reactive force field. The effects of impact velocities and void sizes on the chemical reactions of the CL-20 crystal were discussed. The initial reaction of CL-20 molecules around the voids was analyzed, and the evolution of the formation and breakage of chemical bonds as well as the elementary reactions were also obtained. It is found that under an impact, the CL-20 molecules around the voids first undergo polymerization of the N–O bonds and then breakage of the C–N, N–N, and C–H bonds occurs. Increased void size and impact velocity lead to higher temperature “hot spots” and more intense chemical reactions, but have little effect on the breaking sequence of chemical bonds in the CL-20 molecules.
中文翻译:

含纳米空隙的激波CL-20晶体初始化学反应机理的分子动力学模拟
为了了解多相爆炸性六硝基六氮杂异纤锌矿型结构烷烃(CL-20)的初始化学反应机理,有必要研究这种含纳米空隙晶体的冲击引发机理。在本文中,构造了具有不同空隙尺寸的CL-20超级电池。基于ReaxFF-lg反作用力场,使用分子动力学计算了不同冲击速度引起的化学反应。讨论了冲击速度和空隙尺寸对CL-20晶体化学反应的影响。分析了CL-20分子在空隙周围的初始反应,并获得了化学键形成和断裂的演化以及基本反应。发现受到冲击,空隙周围的CL-20分子首先经历N–O键的聚合,然后发生C–N,N–N和C–H键的断裂。增大的空隙尺寸和撞击速度会导致较高的温度“热点”和更强烈的化学反应,但对CL-20分子中化学键的断裂顺序影响很小。
更新日期:2019-09-18
中文翻译:

含纳米空隙的激波CL-20晶体初始化学反应机理的分子动力学模拟
为了了解多相爆炸性六硝基六氮杂异纤锌矿型结构烷烃(CL-20)的初始化学反应机理,有必要研究这种含纳米空隙晶体的冲击引发机理。在本文中,构造了具有不同空隙尺寸的CL-20超级电池。基于ReaxFF-lg反作用力场,使用分子动力学计算了不同冲击速度引起的化学反应。讨论了冲击速度和空隙尺寸对CL-20晶体化学反应的影响。分析了CL-20分子在空隙周围的初始反应,并获得了化学键形成和断裂的演化以及基本反应。发现受到冲击,空隙周围的CL-20分子首先经历N–O键的聚合,然后发生C–N,N–N和C–H键的断裂。增大的空隙尺寸和撞击速度会导致较高的温度“热点”和更强烈的化学反应,但对CL-20分子中化学键的断裂顺序影响很小。































 京公网安备 11010802027423号
京公网安备 11010802027423号