当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
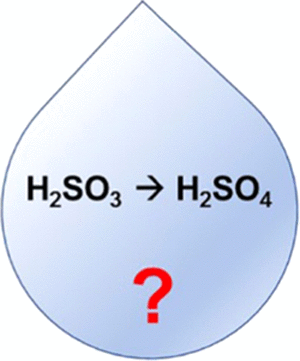
Sulfuric Acid Formation via H2SO3 Oxidation by H2O2 in the Atmosphere
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-09-19 , DOI: 10.1021/acs.jpca.9b05444 Svetlana Shostak 1 , Kitae Kim 2, 3 , Yevhen Horbatenko 1 , Cheol Ho Choi 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2019-09-19 , DOI: 10.1021/acs.jpca.9b05444 Svetlana Shostak 1 , Kitae Kim 2, 3 , Yevhen Horbatenko 1 , Cheol Ho Choi 1
Affiliation
|
With the help of quantum mechanical methods, the formation of H2SO4 by the oxidation of H2SO3 with H2O2 was studied theoretically. Both stepwise and concerted mechanisms were calculated. It was found that the direct oxidation of H2SO3 by H2O2 alone requires prohibitive activation energies of >38.6 kcal/mol. However, the addition of one water molecule exhibits a strong catalytic effect that dramatically reduces the overall reaction barrier to 6.2 (2.3 with PCM) kcal/mol. The deprotonated HSO3– species also reduces the overall reaction barrier to 5.6 (−5.8 with PCM) kcal/mol. Both of these proceed via concerted pathways. On the other hand, the stepwise mechanisms generally produce intermediates with a hydroperoxy group (−O–O–H), which is a result of a nucleophilic attack by the oxygens of H2O2. While studying the catalytic effect of water, a previously unknown hydroperoxy intermediate (HOO)S(OH)3, where sulfur is coordinated with three OH groups, was found. This work also reveals a rearrangement step of another hydroperoxy intermediate (HOO)SO2– to HSO4– that was found in earlier experimental studies. For all of the mechanisms calculated, the final H2SO4 is formed with a significant exothermicity of >60 kcal/mol. In general, even without sunlight, it was found that the formation of sulfuric acid by hydrogen peroxide can occur in a heterogeneous moisturized environment.
中文翻译:

H 2 O 2在大气中通过H 2 SO 3氧化形成硫酸
借助量子力学方法,对H 2 SO 3与H 2 O 2的氧化作用形成H 2 SO 4进行了理论研究。计算了逐步机制和协调机制。已经发现仅H 2 O 2就能直接氧化H 2 SO 3,要求抑制活化能大于38.6 kcal / mol。但是,添加一个水分子会表现出强大的催化作用,从而将总反应势垒显着降低至6.2(使用PCM时为2.3)kcal / mol。脱质子化的HSO 3 –物种还将总反应势垒降低到5.6 kcal / mol(对于PCM为-5.8)。两者都是通过一致的途径进行的。另一方面,逐步机制通常会产生带有氢过氧基团(-O-O-H)的中间体,这是H 2 O 2的氧亲核攻击的结果。在研究水的催化作用时,发现了一个以前未知的氢过氧中间体(HOO)S(OH)3,其中硫与三个OH基团配位。这项工作也揭示了SO另一个氧化氢的中间体(HOO)的重排步骤2 -向HSO 4 -这是在较早的实验研究发现。对于所有计算的机制,最终的H 2形成的SO 4放热度大于60 kcal / mol。通常,即使在没有阳光的情况下,也发现过氧化氢形成硫酸可以在非均质的潮湿环境中发生。
更新日期:2019-09-19
中文翻译:

H 2 O 2在大气中通过H 2 SO 3氧化形成硫酸
借助量子力学方法,对H 2 SO 3与H 2 O 2的氧化作用形成H 2 SO 4进行了理论研究。计算了逐步机制和协调机制。已经发现仅H 2 O 2就能直接氧化H 2 SO 3,要求抑制活化能大于38.6 kcal / mol。但是,添加一个水分子会表现出强大的催化作用,从而将总反应势垒显着降低至6.2(使用PCM时为2.3)kcal / mol。脱质子化的HSO 3 –物种还将总反应势垒降低到5.6 kcal / mol(对于PCM为-5.8)。两者都是通过一致的途径进行的。另一方面,逐步机制通常会产生带有氢过氧基团(-O-O-H)的中间体,这是H 2 O 2的氧亲核攻击的结果。在研究水的催化作用时,发现了一个以前未知的氢过氧中间体(HOO)S(OH)3,其中硫与三个OH基团配位。这项工作也揭示了SO另一个氧化氢的中间体(HOO)的重排步骤2 -向HSO 4 -这是在较早的实验研究发现。对于所有计算的机制,最终的H 2形成的SO 4放热度大于60 kcal / mol。通常,即使在没有阳光的情况下,也发现过氧化氢形成硫酸可以在非均质的潮湿环境中发生。






































 京公网安备 11010802027423号
京公网安备 11010802027423号