当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
I2-Promoted Multicomponent Dicyclization and Ring-Opening Sequences: Direct Synthesis of Benzo[e][1,4]diazepin-3-ones via Dual C–O Bond Cleavage
Organic Letters ( IF 4.9 ) Pub Date : 2019-09-05 , DOI: 10.1021/acs.orglett.9b02789 Xiao Geng 1 , Can Wang 1 , Chun Huang 1 , Peng Zhao 1 , You Zhou 1 , Yan-Dong Wu 1 , An-Xin Wu 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-09-05 , DOI: 10.1021/acs.orglett.9b02789 Xiao Geng 1 , Can Wang 1 , Chun Huang 1 , Peng Zhao 1 , You Zhou 1 , Yan-Dong Wu 1 , An-Xin Wu 1
Affiliation
|
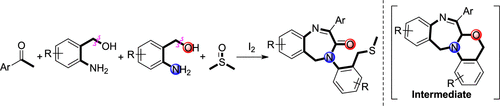
A novel and efficient formal [4 + 2+1] annulation of aryl methyl ketones and 2-aminobenzyl alcohols for the synthesis of benzo[e][1,4]diazepin-3-ones is reported. This reaction successfully affords diverse seven-membered ring lactams via dual C–O bond cleavage. A preliminary mechanistic study showed that a multicomponent dicyclization and ring-opening sequence might occur, with the introduction of methyl sulfide proposed as the last step. This efficient strategy with mild reaction conditions and a broad substrate scope has potential applications in chemistry and medicine.
中文翻译:

I2促进的多组分二环化和开环序列:通过双C–O键裂解直接合成苯并[e] [1,4] diazepin-3-ones
报道了一种新颖有效的芳基甲基酮和2-氨基苄醇的正式[4 + 2 + 1]环合反应,用于合成苯并[ e ] [1,4]二氮杂-3-酮。该反应通过双C-O键裂解成功提供了多种七元环内酰胺。初步的机理研究表明,可能会发生多组分二环化和开环过程,而提议的最后一步是引入甲基硫醚。具有温和的反应条件和广泛的底物范围的这种有效策略在化学和医学中具有潜在的应用。
更新日期:2019-09-06
中文翻译:

I2促进的多组分二环化和开环序列:通过双C–O键裂解直接合成苯并[e] [1,4] diazepin-3-ones
报道了一种新颖有效的芳基甲基酮和2-氨基苄醇的正式[4 + 2 + 1]环合反应,用于合成苯并[ e ] [1,4]二氮杂-3-酮。该反应通过双C-O键裂解成功提供了多种七元环内酰胺。初步的机理研究表明,可能会发生多组分二环化和开环过程,而提议的最后一步是引入甲基硫醚。具有温和的反应条件和广泛的底物范围的这种有效策略在化学和医学中具有潜在的应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号