当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CXCR4‐Enriched Nano‐Trap Targeting CXCL12 in Lung for Early Prevention and Enhanced Photodynamic Therapy of Breast Cancer Metastasis
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-09-06 , DOI: 10.1002/adfm.201905480 Zhaohui Wang 1 , Yi Ma 1 , Han Wang 1 , Fang Wang 1 , Tian Xu 1 , Yueqing Gu 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-09-06 , DOI: 10.1002/adfm.201905480 Zhaohui Wang 1 , Yi Ma 1 , Han Wang 1 , Fang Wang 1 , Tian Xu 1 , Yueqing Gu 1
Affiliation
|
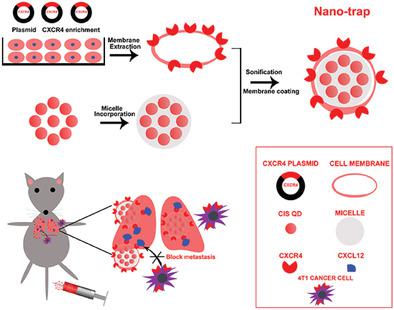
Breast cancer metastasis is strongly correlated with CXCR4‐CXCL12 axis, in which the CXCR4 positive cancer cells are recruited to target organs rich in CXCL12. Although various agents have been developed to inhibit CXCR4, few strategies are reported for targeting and perturbation of CXCL12 to control breast cancer metastasis. Inspired by the increasing popularity of cell membrane (CM)‐derived therapeutics, herein, CXCR4‐enriched 4T1 CMs loaded with copper‐indium‐sulfide quantum dots (QDs) nanoparticles are employed as Nano‐trap to occupy CXCL12 and block breast cancer lung metastasis. CMs fused onto QDs cores faithfully inherit CXCR4 expression of the source cells. CXCR4‐upregulated Nano‐trap binds CXCL12 protein more efficiently than the CXCR4‐silenced counterparts, which effectively abrogate CXCL12‐mediated cancer cell invasion in vitro. In vivo fluorescent imaging reveals preferential distribution of Nano‐trap in lungs with abundant CXCL12 expression. Further interrogation of the in vivo efficacy finds lung metastasis is successfully delayed in breast cancer models pre‐injected with Nano‐trap, which reduces CXCL12 exposure in lung. For the already formed lung metastasis, Nano‐trap can alleviate hypoxia by regulating alpha‐smooth muscle actin, thus improving photodynamic therapy in the metastatic tumor. This proof‐of‐concept study sheds light on exploiting more functionalities of CM proteins for metastasis management.
中文翻译:

靶向CXCL12的富含CXCR4的肺纳米管可用于肺癌的早期预防和增强的光动力治疗。
乳腺癌转移与CXCR4-CXCL12轴密切相关,其中CXCR4阳性癌细胞被募集到富含CXCL12的靶器官。尽管已开发出多种抑制CXCR4的药物,但几乎没有报道靶向和干扰CXCL12来控制乳腺癌转移的策略。受细胞膜(CM)疗法日益普及的启发,本文将富含CXCR4的4T1 CM(载有铜-铟-硫化物量子点(QD)纳米粒子)用作Nano-trap来占据CXCL12并阻止乳腺癌的肺转移。融合到QD核心上的CM忠实地继承了源细胞的CXCR4表达。CXCR4上调的纳米阱比CXCR4沉默的对应物更有效地结合CXCL12蛋白,从而有效地消除了CXCL12介导的癌细胞在体外的侵袭。体内荧光成像显示Nano-trap在肺中具有丰富的CXCL12表达的优先分布。对体内功效的进一步调查发现,在预先注射Nano-trap的乳腺癌模型中,肺转移成功地延迟了,从而减少了CXCL12在肺中的暴露。对于已经形成的肺转移,Nano-trap可通过调节α-平滑肌肌动蛋白来缓解缺氧,从而改善转移瘤的光动力治疗。这项概念验证研究为利用CM蛋白的更多功能进行转移管理提供了启示。减少了肺中CXCL12的暴露。对于已经形成的肺转移,Nano-trap可通过调节α-平滑肌肌动蛋白来缓解缺氧,从而改善转移瘤的光动力治疗。这项概念验证研究为利用CM蛋白的更多功能进行转移管理提供了启示。减少了肺中CXCL12的暴露。对于已经形成的肺转移,Nano-trap可通过调节α-平滑肌肌动蛋白来缓解缺氧,从而改善转移瘤的光动力治疗。这项概念验证研究为利用CM蛋白的更多功能进行转移管理提供了启示。
更新日期:2019-11-06
中文翻译:

靶向CXCL12的富含CXCR4的肺纳米管可用于肺癌的早期预防和增强的光动力治疗。
乳腺癌转移与CXCR4-CXCL12轴密切相关,其中CXCR4阳性癌细胞被募集到富含CXCL12的靶器官。尽管已开发出多种抑制CXCR4的药物,但几乎没有报道靶向和干扰CXCL12来控制乳腺癌转移的策略。受细胞膜(CM)疗法日益普及的启发,本文将富含CXCR4的4T1 CM(载有铜-铟-硫化物量子点(QD)纳米粒子)用作Nano-trap来占据CXCL12并阻止乳腺癌的肺转移。融合到QD核心上的CM忠实地继承了源细胞的CXCR4表达。CXCR4上调的纳米阱比CXCR4沉默的对应物更有效地结合CXCL12蛋白,从而有效地消除了CXCL12介导的癌细胞在体外的侵袭。体内荧光成像显示Nano-trap在肺中具有丰富的CXCL12表达的优先分布。对体内功效的进一步调查发现,在预先注射Nano-trap的乳腺癌模型中,肺转移成功地延迟了,从而减少了CXCL12在肺中的暴露。对于已经形成的肺转移,Nano-trap可通过调节α-平滑肌肌动蛋白来缓解缺氧,从而改善转移瘤的光动力治疗。这项概念验证研究为利用CM蛋白的更多功能进行转移管理提供了启示。减少了肺中CXCL12的暴露。对于已经形成的肺转移,Nano-trap可通过调节α-平滑肌肌动蛋白来缓解缺氧,从而改善转移瘤的光动力治疗。这项概念验证研究为利用CM蛋白的更多功能进行转移管理提供了启示。减少了肺中CXCL12的暴露。对于已经形成的肺转移,Nano-trap可通过调节α-平滑肌肌动蛋白来缓解缺氧,从而改善转移瘤的光动力治疗。这项概念验证研究为利用CM蛋白的更多功能进行转移管理提供了启示。






































 京公网安备 11010802027423号
京公网安备 11010802027423号