Communications Chemistry ( IF 5.9 ) Pub Date : 2019-09-05 , DOI: 10.1038/s42004-019-0208-2 Pei Fan , Chuan Wang
|
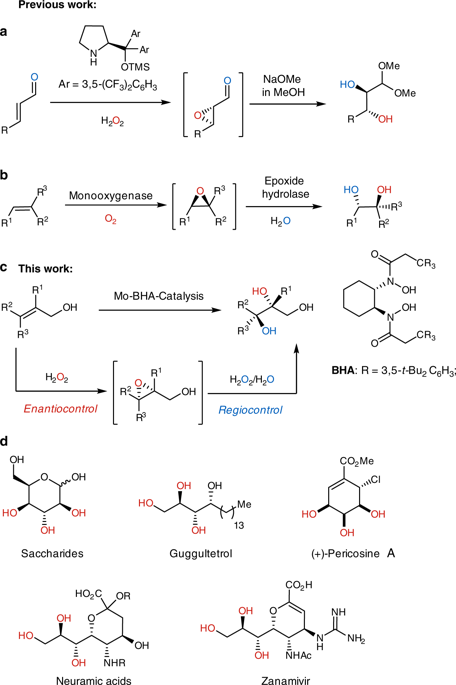
Asymmetric dihydroxylation of alkenes is one of the fundamental reactions in organic synthesis, but the anti-dihydroxylation is much less developed than its syn-variant. Here we report a highly enantio- and diastereoselective anti-dihydroxylation of allylic alcohols by using a chiral molybdenum-bishydroxamic acid complex as catalyst and environmentally benign hydrogen peroxide as oxidant. This reaction enables the construction of the 1,2,3-triol structural unit in high enantio- and diastereocontrol starting from simple allylic alcohol precursors. Our reaction complements the Sharpless dihydroxylation not only in its diastereoselectivity, but also in regiocontrol. The mechanistic studies indicate that this dihydroxylation reaction consists of an initial enantioselective epoxidation and the following in situ regioselective ring opening, both of which are promoted by the molybdenum-catalyst.
中文翻译:

钼催化的烯丙基醇的不对称抗二羟基化
烯烃的不对称二羟基化是有机合成中的基本反应之一,但抗二羟基化的发展远不及其顺式变体。在这里我们报告了高度对映体和非对映体选择性的抗使用手性钼-双异羟肟酸络合物作为催化剂,对环境无害的过氧化氢作为氧化剂,对烯丙醇进行二羟基化。该反应使得能够从简单的烯丙基醇前体开始以高对映体和非对映体的方式构造1,2,3-三醇结构单元。我们的反应不仅在非对映选择性上还补充了Sharpless二羟基化反应,而且在区域控制方面也起到了补充作用。机理研究表明,该二羟基化反应由初始的对映选择性环氧化和随后的原位区域选择性开环组成,两者均由钼催化剂促进。































 京公网安备 11010802027423号
京公网安备 11010802027423号