Nature Communications ( IF 14.7 ) Pub Date : 2019-09-05 , DOI: 10.1038/s41467-019-11992-2 Kun Jiang 1 , Seoin Back 2 , Austin J Akey 3 , Chuan Xia 1 , Yongfeng Hu 4 , Wentao Liang 5 , Diane Schaak 1 , Eli Stavitski 6 , Jens K Nørskov 2, 7 , Samira Siahrostami 2, 8 , Haotian Wang 1, 9
|
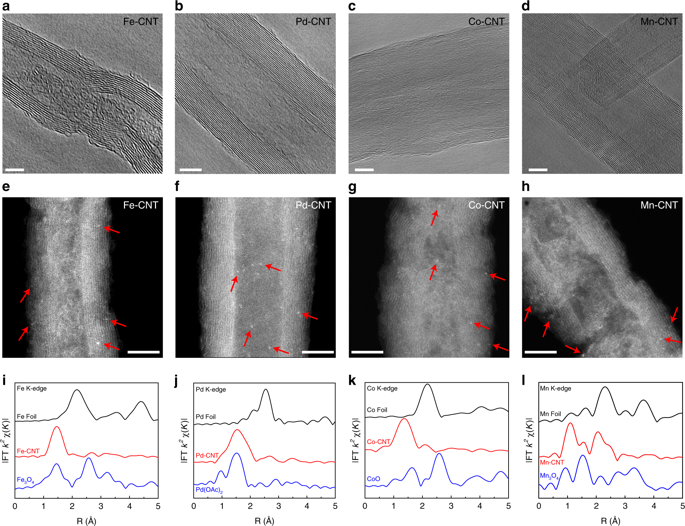
Shifting electrochemical oxygen reduction towards 2e– pathway to hydrogen peroxide (H2O2), instead of the traditional 4e– to water, becomes increasingly important as a green method for H2O2 generation. Here, through a flexible control of oxygen reduction pathways on different transition metal single atom coordination in carbon nanotube, we discovered Fe-C-O as an efficient H2O2 catalyst, with an unprecedented onset of 0.822 V versus reversible hydrogen electrode in 0.1 M KOH to deliver 0.1 mA cm−2 H2O2 current, and a high H2O2 selectivity of above 95% in both alkaline and neutral pH. A wide range tuning of 2e–/4e– ORR pathways was achieved via different metal centers or neighboring metalloid coordination. Density functional theory calculations indicate that the Fe-C-O motifs, in a sharp contrast to the well-known Fe-C-N for 4e–, are responsible for the H2O2 pathway. This iron single atom catalyst demonstrated an effective water disinfection as a representative application.
中文翻译:

在过渡金属单原子配位上高选择性氧还原成过氧化氢。
将电化学氧还原转向 2e (生成过氧化氢 (H 2 O 2 )),而不是传统的 4e (生成水),作为 H 2 O 2生成的绿色方法变得越来越重要。在这里,通过灵活控制碳纳米管中不同过渡金属单原子配位的氧还原途径,我们发现Fe-CO作为一种高效的H 2 O 2催化剂,与0.1 M KOH中的可逆氢电极相比,具有前所未有的0.822 V起始电压提供0.1 mA cm -2 H 2 O 2电流,以及在碱性和中性pH值下均高于95%的高H 2 O 2选择性。通过不同的金属中心或邻近的类金属配位实现了2e – /4e – ORR 途径的大范围调整。密度泛函理论计算表明,与众所周知的 4e –的 Fe-CN 基序形成鲜明对比的是,Fe-CO 基序负责 H 2 O 2途径。这种铁单原子催化剂作为代表性应用证明了有效的水消毒。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号