当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis and Characterization of Robust MoS2 and S Cathode for Advanced Li–S Battery: Combined Experimental and Theoretical Studies
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-09-23 , DOI: 10.1021/acsami.9b11967
Akhil Mammoottil Abraham , Sanoop Palakkathodi Kammampata , Sathish Ponnurangam , Venkataraman Thangadurai
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-09-23 , DOI: 10.1021/acsami.9b11967
Akhil Mammoottil Abraham , Sanoop Palakkathodi Kammampata , Sathish Ponnurangam , Venkataraman Thangadurai
|
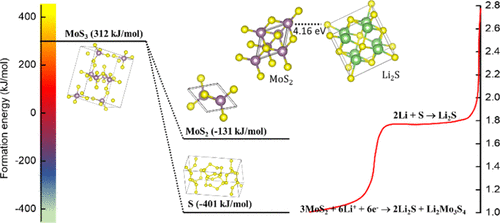
Here, we report that in situ MoS2 and S cathodes (MGC) prepared by simple decomposition of (NH4)2MoS4 facilitate direct formation of Li2S and suppress the long-term problem associated with polysulphide shuttling in Li–S batteries. For comparison, we prepared ex situ MoS2 and S cathodes (EMS) with a similar S/MoS2 mole ratio to that of in situ-prepared cathodes. Discharge capacity of EMS cathodes dropped by 80% after first few cycles, while assembled MGC cells demonstrated an initial discharge capacity of 1649 mA h/g, achieving close to theoretical capacity of elemental sulfur (1675 mA h/g) at C/3 and a reversible capacity of 1500 mA h/g was obtained in further cycles. The MoS2 nanostructure evolution after initial discharge helped in extending the cycle life of assembled cells even at a high C rate. Density functional theory (DFT) calculation was performed to understand the structural stability of intermediate MoS3 and possible electrochemical reactions pertaining to Li+ insertion in MoS2 and S. Based on DFT studies, MoS3 undergoes stoichiometric decomposition to stable MoS2 and S. Furthermore, electrochemical analysis confirmed the redox activity of MoS2 and S at 1.3 and 1.8 V against Li/Li+, respectively.
中文翻译:

先进的Li-S电池稳健的MoS 2和S阴极的高效合成和表征:结合实验和理论研究
在这里,我们报告说,通过简单分解(NH 4)2 MoS 4制备的原位MoS 2和S阴极(MGC)有助于Li 2 S的直接形成,并抑制了与Li–S电池中多硫化物穿梭有关的长期问题。为了进行比较,我们准备了具有类似S / MoS 2的异位MoS 2和S阴极(EMS)摩尔比与原位制备的阴极的摩尔比。在最初的几个循环后,EMS阴极的放电容量下降了80%,而组装的MGC电池的初始放电容量为1649 mA h / g,在C / 3和C / 3时达到了接近元素硫的理论容量(1675 mA h / g)。在进一步的循环中获得了1500 mA h / g的可逆容量。初始放电后MoS 2纳米结构的演变有助于延长组装电池的循环寿命,即使在高C速率下也是如此。进行密度泛函理论(DFT)计算以了解中间MoS 3的结构稳定性以及与Li +插入MoS 2和S中有关的可能的电化学反应。基于DFT研究,MoS 3进行化学计量分解,生成稳定的MoS 2和S。此外,电化学分析证实,MoS 2和S分别在1.3和1.8 V下对Li / Li +的氧化还原活性。
更新日期:2019-09-23
中文翻译:

先进的Li-S电池稳健的MoS 2和S阴极的高效合成和表征:结合实验和理论研究
在这里,我们报告说,通过简单分解(NH 4)2 MoS 4制备的原位MoS 2和S阴极(MGC)有助于Li 2 S的直接形成,并抑制了与Li–S电池中多硫化物穿梭有关的长期问题。为了进行比较,我们准备了具有类似S / MoS 2的异位MoS 2和S阴极(EMS)摩尔比与原位制备的阴极的摩尔比。在最初的几个循环后,EMS阴极的放电容量下降了80%,而组装的MGC电池的初始放电容量为1649 mA h / g,在C / 3和C / 3时达到了接近元素硫的理论容量(1675 mA h / g)。在进一步的循环中获得了1500 mA h / g的可逆容量。初始放电后MoS 2纳米结构的演变有助于延长组装电池的循环寿命,即使在高C速率下也是如此。进行密度泛函理论(DFT)计算以了解中间MoS 3的结构稳定性以及与Li +插入MoS 2和S中有关的可能的电化学反应。基于DFT研究,MoS 3进行化学计量分解,生成稳定的MoS 2和S。此外,电化学分析证实,MoS 2和S分别在1.3和1.8 V下对Li / Li +的氧化还原活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号