当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-Symmetrical Bis-Azine Biaryls from Chloroazines: A Strategy Using Phosphorus Ligand-Coupling
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-04 , DOI: 10.1021/jacs.9b08504 Benjamin T Boyle 1 , Michael C Hilton 1 , Andrew McNally 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-04 , DOI: 10.1021/jacs.9b08504 Benjamin T Boyle 1 , Michael C Hilton 1 , Andrew McNally 1
Affiliation
|
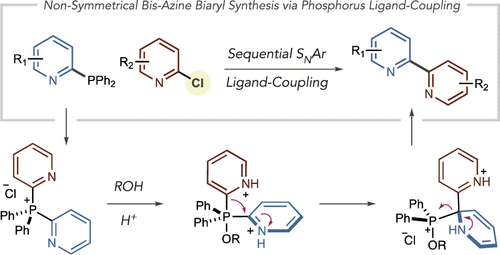
Distinct approaches to synthesize bis-azine biaryls are in demand as these compounds have multiple applications in the chemical sciences and are challenging targets for metal-catalyzed cross-coupling reactions. Most approaches focus on developing new reagents as the formal nucleophilic coupling partner that can function in metal-catalyzed processes. We present an alternative approach using pyridine and diazine phosphines as nucleophilic partners and chloroazines where the heterobiaryl bond is formed via a tandem SNAr-phosphorus ligand-coupling sequence. The heteroaryl phosphines are prepared from chloroazines and are bench-stable solids. A range of bis-azine biaryls can be formed from abundant chloroazines using this strategy that would be challenging using traditional approaches. A one-pot cross-electrophile coupling of two chloroazines is feasible, and we also compared the phosphorus-mediated strategy with metal-catalyzed coupling reactions to show advantages and compatibility.
中文翻译:

来自氯嗪的非对称双氮杂二芳基化合物:一种使用磷配体偶联的策略
需要独特的方法来合成双嗪联芳基化合物,因为这些化合物在化学科学中有多种应用,并且是金属催化交叉偶联反应的挑战性目标。大多数方法侧重于开发新试剂,作为可以在金属催化过程中发挥作用的正式亲核偶联伙伴。我们提出了一种替代方法,使用吡啶和二嗪膦作为亲核伙伴和氯嗪,其中杂联芳键是通过串联 SNAr-磷配体偶联序列形成的。杂芳基膦由氯嗪制备并且是长期稳定的固体。使用这种策略可以从丰富的氯嗪中形成一系列双嗪联芳基化合物,这对于使用传统方法来说是具有挑战性的。
更新日期:2019-09-04
中文翻译:

来自氯嗪的非对称双氮杂二芳基化合物:一种使用磷配体偶联的策略
需要独特的方法来合成双嗪联芳基化合物,因为这些化合物在化学科学中有多种应用,并且是金属催化交叉偶联反应的挑战性目标。大多数方法侧重于开发新试剂,作为可以在金属催化过程中发挥作用的正式亲核偶联伙伴。我们提出了一种替代方法,使用吡啶和二嗪膦作为亲核伙伴和氯嗪,其中杂联芳键是通过串联 SNAr-磷配体偶联序列形成的。杂芳基膦由氯嗪制备并且是长期稳定的固体。使用这种策略可以从丰富的氯嗪中形成一系列双嗪联芳基化合物,这对于使用传统方法来说是具有挑战性的。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号