当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A base-promoted cascade reaction of α,β-unsaturated N-tosylhydrazones with o-hydroxybenzyl alcohols: highly regioselective synthesis of N-sec-alkylpyrazoles.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-09-25 , DOI: 10.1039/c9ob01780a Lian-Mei Chen 1 , Juan Zhao , An-Jie Xia , Xiao-Qiang Guo , Ya Gan , Chuang Zhou , Zai-Jun Yang , Jun Yang , Tai-Ran Kang
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2019-09-25 , DOI: 10.1039/c9ob01780a Lian-Mei Chen 1 , Juan Zhao , An-Jie Xia , Xiao-Qiang Guo , Ya Gan , Chuang Zhou , Zai-Jun Yang , Jun Yang , Tai-Ran Kang
Affiliation
|
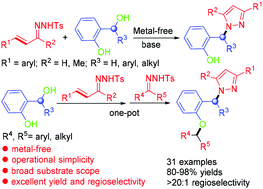
An efficient method for the synthesis of N-sec-alkylpyrazoles through a base-promoted cascade cyclization/Michael addition reaction of α,β-unsaturated N-tosylhydrazones with ortho-hydroxybenzyl alcohols has been developed. The desired products containing di- or triaryl groups at the same carbon atom were afforded in good to excellent yields with excellent regioselectivities (>20 : 1). Moreover, a three-component reaction of ortho-hydroxybenzyl alcohols, α,β-unsaturated N-tosylhydrazones and saturated N-tosylhydrazones also took place to afford pyrazoles in good yields. This reaction offers a new route to triarylmethanes with a simple operation and is applicable for large-scale synthesis.
中文翻译:

α,β-不饱和N-甲苯磺酰hydr与邻羟基苄醇的碱促进的级联反应:N-仲烷基吡唑的高度区域选择性合成。
已经开发了一种通过α,β-不饱和N-甲苯磺酰with与邻羟基苄醇的碱促进的级联环化/ Michael加成反应合成N-仲烷基吡唑的有效方法。所提供的具有在相同碳原子上的二芳基或三芳基基团的期望产物以良好至优异的产率以及优异的区域选择性(> 20∶1)得到。此外,还进行了邻羟基苄醇,α,β-不饱和N-甲苯磺酰azo和饱和N-甲苯磺酰nes的三组分反应,以高收率得到吡唑。该反应以简单的操作提供了一条新的途径制备三芳基甲烷,适用于大规模合成。
更新日期:2019-09-12
中文翻译:

α,β-不饱和N-甲苯磺酰hydr与邻羟基苄醇的碱促进的级联反应:N-仲烷基吡唑的高度区域选择性合成。
已经开发了一种通过α,β-不饱和N-甲苯磺酰with与邻羟基苄醇的碱促进的级联环化/ Michael加成反应合成N-仲烷基吡唑的有效方法。所提供的具有在相同碳原子上的二芳基或三芳基基团的期望产物以良好至优异的产率以及优异的区域选择性(> 20∶1)得到。此外,还进行了邻羟基苄醇,α,β-不饱和N-甲苯磺酰azo和饱和N-甲苯磺酰nes的三组分反应,以高收率得到吡唑。该反应以简单的操作提供了一条新的途径制备三芳基甲烷,适用于大规模合成。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号