Biomaterials ( IF 12.8 ) Pub Date : 2019-09-05 , DOI: 10.1016/j.biomaterials.2019.119469 Liqin Hu 1 , Ziyang Cao 2 , Leilei Ma 1 , Zhongqiu Liu 1 , Guochao Liao 1 , Junxia Wang 2 , Song Shen 3 , Dongdong Li 4 , Xianzhu Yang 5
|
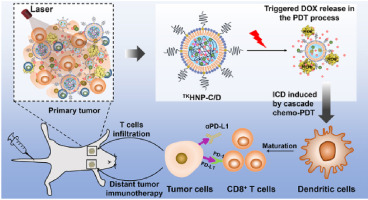
Checkpoint inhibitors, such as anti-PD-1/PD-L1 antibodies, have been proven as a promising type of immunotherapy in a number of cancers, but the relatively low response rates limit their scope of clinical application. Here, we report the use of cascade chemo-photodynamic therapy (chemo-PDT) with reactive oxygen species (ROS)-sensitive lipid-polymer hybrid nanoparticles TKHNP-C/D to potentiate the antitumor efficacy of anti-PD-L1 antibody (aPD-L1). Under light irradiation, TKHNP-C/D not only induced photodynamic therapy (PDT) but also boosted intracellular DOX release via the rapid degradation of its hydrophobic core, promoting an efficient cascade of chemo-PDT to inhibit tumor growth by a single treatment. More importantly, the cascade chemo-PDT could evoke anticancer immune responses and efficiently synergize with aPD-L1 to generate an abscopal effect, which could simultaneously inhibit primary and distant tumor growth.
中文翻译:

ROS反应的纳米载体介导的级联化学光动力疗法增强了检查站的免疫治疗。
检查点抑制剂,例如抗-PD-1 / PD-L1抗体,已被证明是许多癌症中一种有前途的免疫疗法,但是相对较低的应答率限制了其临床应用范围。在这里,我们报告了级联化学光动力疗法(chemo-PDT)与活性氧(ROS)敏感的脂质-聚合物杂化纳米颗粒TK HNP-C / D的使用,以增强抗-PD-L1抗体的抗肿瘤功效( aPD-L1)。在光照射下,TK HNP-C / D不仅诱导了光动力疗法(PDT),而且还通过以下途径增强了细胞内DOX的释放:疏水核心的快速降解,通过单一治疗促进了化学PDT的有效级联,从而抑制了肿瘤的生长。更重要的是,级联化学PDT可以引起抗癌免疫反应,并与aPD-L1有效协同产生明显的效应,从而可以同时抑制原发性和远处的肿瘤生长。






























 京公网安备 11010802027423号
京公网安备 11010802027423号