当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Deuterium Incorporation within Metabolically Stable β-Amino C–H Bonds of Drug Molecules
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-03 , DOI: 10.1021/jacs.9b08662 Yejin Chang 1 , Ahmet Yesilcimen 1 , Min Cao 1 , Yuyang Zhang 1 , Bochao Zhang 1 , Jessica Z Chan 1 , Masayuki Wasa 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-03 , DOI: 10.1021/jacs.9b08662 Yejin Chang 1 , Ahmet Yesilcimen 1 , Min Cao 1 , Yuyang Zhang 1 , Bochao Zhang 1 , Jessica Z Chan 1 , Masayuki Wasa 1
Affiliation
|
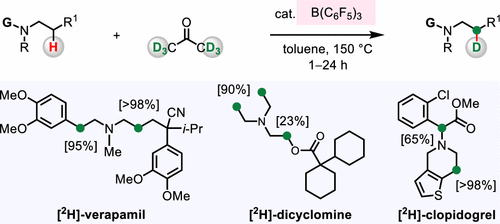
An efficient deuteration process of beta-amino C-H bonds in various N-alkylamine-based pharmaceutical compounds has been developed. Catalytic reactions begin with the action of Lewis acidic B(C6F5)3 and Brønsted basic N-alkylamine, converting a drug molecule into the corresponding enamine. The acid/base catalysts also promote the dedeuteration of acetone-d6 to afford a deuterated ammonium ion. Ensuing deuteration of the enamine then leads to the formation of beta-deuterated bioactive amines with up to 99% deuterium incorporation.
中文翻译:

药物分子代谢稳定的 β-氨基 C-H 键中的催化氘掺入
已开发出各种基于 N-烷基胺的药物化合物中 β-氨基 CH 键的有效氘化过程。催化反应开始于路易斯酸性 B(C6F5)3 和布朗斯台德碱性 N-烷基胺的作用,将药物分子转化为相应的烯胺。酸/碱催化剂还促进丙酮-d6 的氘化以提供氘化铵离子。随后烯胺的氘化会导致β-氘化生物活性胺的形成,其中氘掺入量高达 99%。
更新日期:2019-09-03
中文翻译:

药物分子代谢稳定的 β-氨基 C-H 键中的催化氘掺入
已开发出各种基于 N-烷基胺的药物化合物中 β-氨基 CH 键的有效氘化过程。催化反应开始于路易斯酸性 B(C6F5)3 和布朗斯台德碱性 N-烷基胺的作用,将药物分子转化为相应的烯胺。酸/碱催化剂还促进丙酮-d6 的氘化以提供氘化铵离子。随后烯胺的氘化会导致β-氘化生物活性胺的形成,其中氘掺入量高达 99%。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号