Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The role of anthrax toxin protein receptor 1 as a new mechanosensor molecule and its mechanotransduction in BMSCs under hydrostatic pressure.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-02 , DOI: 10.1038/s41598-019-49100-5 Baixiang Cheng 1 , Yanzheng Liu 1 , Ying Zhao 1 , Qiang Li 1 , Yanli Liu 1 , Junjun Wang 1 , Yongjin Chen 1 , Min Zhang 1
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-02 , DOI: 10.1038/s41598-019-49100-5 Baixiang Cheng 1 , Yanzheng Liu 1 , Ying Zhao 1 , Qiang Li 1 , Yanli Liu 1 , Junjun Wang 1 , Yongjin Chen 1 , Min Zhang 1
Affiliation
|
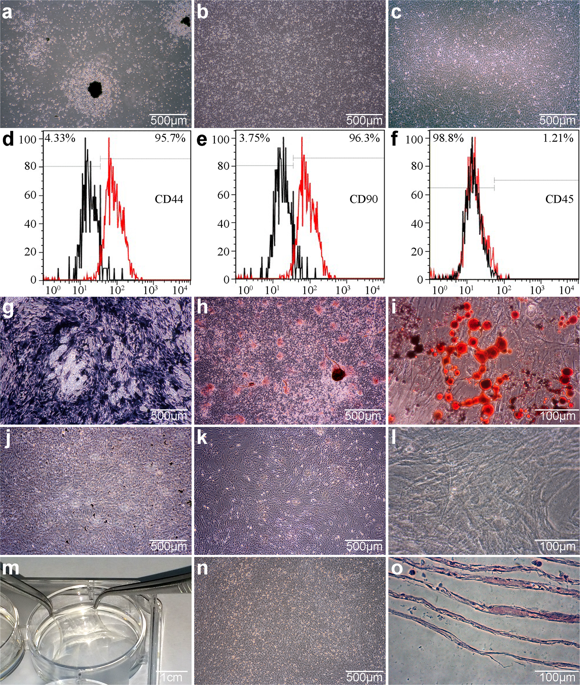
Anthrax toxin protein receptor (ANTXR) 1 has many similarities to integrin and is regarded in some respects as a single-stranded integrin protein. However, it is not clear whether ANTXR1 responds to mechanical signals secondary to the activation of integrins or whether it is a completely new, independent and previously undiscovered mechanosensor that responds to an undefined subset of mechanical signaling molecules. Our study demonstrates that ANTXR1 is a novel mechanosensor on the cell membrane, acting independently from the classical mechanoreceptor molecule integrinβ1. We show that bone marrow stromal cells (BMSCs) respond to the hydrostatic pressure towards chondrogenic differentiation partly through the glycogen synthase kinase (GSK) 3β/β-Catenin signaling pathway, which can be partly regulated by ANTXR1 and might be related to the direct binding between ANTXR1 and low-density lipoprotein receptor-related protein (LRP) 5/6. In addition, ANTXR1 specifically activates Smad2 and upregulates Smad4 expression to facilitate the transport of activated Smad2 to the nucleus to regulate chondrogenesis, which might be related to the direct binding between ANTXR1 and Actin/Fascin1. We also demonstrate that ANTXR1 binds to some extent with integrinβ1, but this interaction does not affect the expression and function of either protein under pressure. Thus, we conclude that ANTXR1 plays a crucial role in BMSC mechanotransduction and controls specific signaling pathways that are distinct from those of integrin to influence the chondrogenic responses of BMSCs under hydrostatic pressure.
中文翻译:

炭疽毒素蛋白受体1作为一种新的机械传感器分子的作用及其在静水压力下在骨髓间充质干细胞中的机械转导。
炭疽毒素蛋白受体(ANTXR)1与整联蛋白有很多相似之处,在某些方面被视为单链整联蛋白。但是,尚不清楚ANTXR1是否响应整合素激活后的机械信号,或者它是否是对机械信号分子的未定义子集作出响应的全新,独立且以前未被发现的机械传感器。我们的研究表明,ANTXR1是细胞膜上的新型机械传感器,其作用与经典的机械受体分子整联蛋白β1独立。我们显示,骨髓基质细胞(BMSC)响应部分软骨部分通过糖原合酶激酶(GSK)3β/β-Catenin信号传导途径向软骨分化的静水压力,它可能部分受ANTXR1调节,可能与ANTXR1和低密度脂蛋白受体相关蛋白(LRP)5/6之间的直接结合有关。此外,ANTXR1特异性激活Smad2,并上调Smad4表达,以促进活化的Smad2转运至细胞核,从而调节软骨形成,这可能与ANTXR1和Actin / Fascin1之间的直接结合有关。我们还证明了ANTXR1在某种程度上与整联蛋白β1结合,但是这种相互作用并不影响任何一种蛋白质在压力下的表达和功能。因此,我们得出结论,ANTXR1在BMSC机械转导中起着至关重要的作用,并控制与整联蛋白不同的特定信号传导途径,以影响在静水压力下BMSC的成软骨反应。
更新日期:2019-09-03
中文翻译:

炭疽毒素蛋白受体1作为一种新的机械传感器分子的作用及其在静水压力下在骨髓间充质干细胞中的机械转导。
炭疽毒素蛋白受体(ANTXR)1与整联蛋白有很多相似之处,在某些方面被视为单链整联蛋白。但是,尚不清楚ANTXR1是否响应整合素激活后的机械信号,或者它是否是对机械信号分子的未定义子集作出响应的全新,独立且以前未被发现的机械传感器。我们的研究表明,ANTXR1是细胞膜上的新型机械传感器,其作用与经典的机械受体分子整联蛋白β1独立。我们显示,骨髓基质细胞(BMSC)响应部分软骨部分通过糖原合酶激酶(GSK)3β/β-Catenin信号传导途径向软骨分化的静水压力,它可能部分受ANTXR1调节,可能与ANTXR1和低密度脂蛋白受体相关蛋白(LRP)5/6之间的直接结合有关。此外,ANTXR1特异性激活Smad2,并上调Smad4表达,以促进活化的Smad2转运至细胞核,从而调节软骨形成,这可能与ANTXR1和Actin / Fascin1之间的直接结合有关。我们还证明了ANTXR1在某种程度上与整联蛋白β1结合,但是这种相互作用并不影响任何一种蛋白质在压力下的表达和功能。因此,我们得出结论,ANTXR1在BMSC机械转导中起着至关重要的作用,并控制与整联蛋白不同的特定信号传导途径,以影响在静水压力下BMSC的成软骨反应。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号