Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2019-09-02 , DOI: 10.1016/j.jinorgbio.2019.110820 Yun-Liang Zhang 1 , Cai-Xing Deng 2 , Wen-Feng Zhou 2 , Liu-Yan Zhou 2 , Qian-Qian Cao 2 , Wen-Ying Shen 2 , Hong Liang 2 , Zhen-Feng Chen 2
|
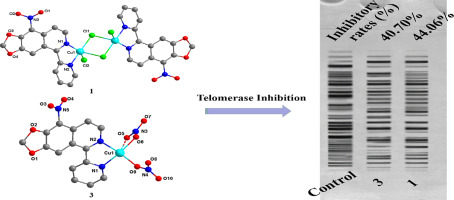
Seven Cu(II) complexes with 5-pyridin-2-yl-[1,3]dioxolo[4,5-g]isoquinoline derivatives as ligands: [Cu2(L1)2Cl4] (1), [Cu(L2)Cl2] (2), [Cu(L1)(NO3)2] (3), [Cu(L2)(NO3)2] (4), [Cu(L3)Cl2] (5), [Cu(L3)Br2] (6) and [Cu(L3)(NO3)2] (7){L1=9-nitro-5-pyridin-2-yl-[1,3]dioxolo[4,5-g]isoquinoline, L2=4-nitro-5-pyridin-2-yl-[1,3]dioxolo[4,5-g]isoquinoline, L3=9-bromo-5-pyridin-2-yl-[1,3]dioxolo[4,5-g]isoquinoline}, were synthesized and characterized. Their in vitro anticancer activities against T-24, MGC-80-3, HeLa, Hep-G2, A549 and SK-OV-3 were evaluated. Compared with their corresponding ligands, most of these complexes exhibited enhanced anticancer activities in contrast to their corresponding ligands and copper salt. Among them, complexes 1 and 3 displayed selective cytotoxicity to HeLa cells comparing with normal liver cell HL-7702, with IC50 values of 5.03 ± 1.20 μM and 10.05 ± 0.52 μM, respectively. Complexes 1 and 3 inhibited telomerase activity by interacting with c-myc promoter elements, and therefore exerted their antitumor activity. Furthermore, complexes 1 and 3 could trigger cell apoptosis via disruption of mitochondrial pathway through notably increased reactive oxygen species (ROS) levels, loss of mitochondrial membrane potential (Δψm), increase of the cytochrome c and apaf-1, decrease of bcl-2, and activation of caspases 3/9. Complexes 1 and 3 exhibited enhanced cytotoxicity, presenting synergetic effect after the ligands coordinated to copper(II) center.
中文翻译:

含5-吡啶-2-基-[1,3]二氧杂环[4,5-g]异喹啉衍生物的铜(II)配合物的合成及体外抗肿瘤活性评估。
七个以5-吡啶-2-基-[1,3] dioxolo [4,5- g ]异喹啉衍生物为配体的Cu(II)配合物:[Cu 2(L 1)2 Cl 4 ](1),[Cu (L 2)Cl 2 ](2),[Cu(L 1)(NO 3)2 ](3),[Cu(L 2)(NO 3)2 ](4),[Cu(L 3)Cl 2 ](5),[Cu(L 3)Br 2](6)和[Cu(L 3)(NO 3)2 ](7){ L 1 = 9-硝基-5-吡啶-2--2-基-[1,3]二氧代[4,5- g ]异喹啉,L 2 = 4-硝基-5-吡啶-2-基-[1,3]二氧杂环戊[4,5- g ]异喹啉,L 3 = 9-溴-5-吡啶-2-基-[1,3合成并表征了] dioxolo [4,5- g ]异喹啉}。他们的体外评估了对T-24,MGC-80-3,HeLa,Hep-G2,A549和SK-OV-3的抗癌活性。与它们相应的配体相比,与它们相应的配体和铜盐相比,大多数这些配合物表现出增强的抗癌活性。其中,复合物1和3与正常肝细胞HL-7702相比,对HeLa细胞表现出选择性的细胞毒性,IC 50值分别为5.03±1.20μM和10.05±0.52μM。复合物1和3通过与c-myc启动子元件相互作用抑制端粒酶活性,因此发挥其抗肿瘤活性。此外,复合物1和3可以经由通过显着增加的活性氧线粒体途径的破坏触发细胞凋亡(ROS)水平,线粒体膜电位(Δψ的损失米),细胞色素的增加Ç和APAF-1,BCL-2的减少,和胱天蛋白酶的活化3/9。配合物1和3表现出增强的细胞毒性,在配体配位至铜(II)中心后表现出协同作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号