Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CENP-C unwraps the human CENP-A nucleosome through the H2A C-terminal tail.
EMBO Reports ( IF 6.5 ) Pub Date : 2019-09-02 , DOI: 10.15252/embr.201948913
Ahmad Ali-Ahmad 1 , Silvija Bilokapić 2 , Ingmar B Schäfer 3 , Mario Halić 2 , Nikolina Sekulić 1, 4
EMBO Reports ( IF 6.5 ) Pub Date : 2019-09-02 , DOI: 10.15252/embr.201948913
Ahmad Ali-Ahmad 1 , Silvija Bilokapić 2 , Ingmar B Schäfer 3 , Mario Halić 2 , Nikolina Sekulić 1, 4
Affiliation
|
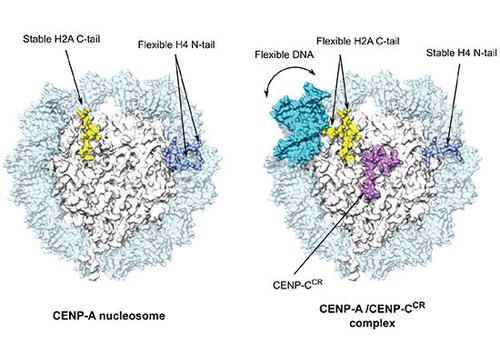
Centromeres are defined epigenetically by nucleosomes containing the histone H3 variant CENP-A, upon which the constitutive centromere-associated network of proteins (CCAN) is built. CENP-C is considered to be a central organizer of the CCAN. We provide new molecular insights into the structure of human CENP-A nucleosomes, in isolation and in complex with the CENP-C central region (CENP-CCR ), the main CENP-A binding module of human CENP-C. We establish that the short αN helix of CENP-A promotes DNA flexibility at the nucleosome ends, independently of the sequence it wraps. Furthermore, we show that, in vitro, two regions of human CENP-C (CENP-CCR and CENP-Cmotif ) both bind exclusively to the CENP-A nucleosome. We find CENP-CCR to bind with high affinity due to an extended hydrophobic area made up of CENP-AV 532 and CENP-AV 533 . Importantly, we identify two key conformational changes within the CENP-A nucleosome upon CENP-C binding. First, the loose DNA wrapping of CENP-A nucleosomes is further exacerbated, through destabilization of the H2A C-terminal tail. Second, CENP-CCR rigidifies the N-terminal tail of H4 in the conformation favoring H4K20 monomethylation, essential for a functional centromere.
中文翻译:

CENP-C 通过 H2A C 末端尾部解开人类 CENP-A 核小体。
着丝粒由含有组蛋白 H3 变体 CENP-A 的核小体进行表观遗传学定义,在此基础上构建了组成型着丝粒相关蛋白网络 (CCAN)。 CENP-C 被认为是 CCAN 的中央组织者。我们对人类 CENP-A 核小体的结构提供了新的分子见解,该核小体与 CENP-C 中心区 (CENP-CCR ) 是分离的和复合的,CENP-CCR 是人类 CENP-C 的主要 CENP-A 结合模块。我们确定 CENP-A 的短 αN 螺旋可促进核小体末端的 DNA 灵活性,与其包裹的序列无关。此外,我们表明,在体外,人类 CENP-C 的两个区域(CENP-CCR 和 CENP-Cmotif )都专门与 CENP-A 核小体结合。我们发现 CENP-CCR 由于由 CENP-AV 532 和 CENP-AV 533 组成的扩展疏水区域而以高亲和力结合。重要的是,我们确定了 CENP-C 结合后 CENP-A 核小体内的两个关键构象变化。首先,H2A C 末端尾部的不稳定进一步加剧了 CENP-A 核小体 DNA 的松散包裹。其次,CENP-CCR 使 H4 的 N 末端尾部刚性化,形成有利于 H4K20 单甲基化的构象,这对于功能性着丝粒至关重要。
更新日期:2019-10-04
中文翻译:

CENP-C 通过 H2A C 末端尾部解开人类 CENP-A 核小体。
着丝粒由含有组蛋白 H3 变体 CENP-A 的核小体进行表观遗传学定义,在此基础上构建了组成型着丝粒相关蛋白网络 (CCAN)。 CENP-C 被认为是 CCAN 的中央组织者。我们对人类 CENP-A 核小体的结构提供了新的分子见解,该核小体与 CENP-C 中心区 (CENP-CCR ) 是分离的和复合的,CENP-CCR 是人类 CENP-C 的主要 CENP-A 结合模块。我们确定 CENP-A 的短 αN 螺旋可促进核小体末端的 DNA 灵活性,与其包裹的序列无关。此外,我们表明,在体外,人类 CENP-C 的两个区域(CENP-CCR 和 CENP-Cmotif )都专门与 CENP-A 核小体结合。我们发现 CENP-CCR 由于由 CENP-AV 532 和 CENP-AV 533 组成的扩展疏水区域而以高亲和力结合。重要的是,我们确定了 CENP-C 结合后 CENP-A 核小体内的两个关键构象变化。首先,H2A C 末端尾部的不稳定进一步加剧了 CENP-A 核小体 DNA 的松散包裹。其次,CENP-CCR 使 H4 的 N 末端尾部刚性化,形成有利于 H4K20 单甲基化的构象,这对于功能性着丝粒至关重要。

































 京公网安备 11010802027423号
京公网安备 11010802027423号