当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of novel diazaspiro[4.5]decan-1-one derivatives as potential chitin synthase inhibitors and antifungal agents.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-08-31 , DOI: 10.1016/j.ejmech.2019.111669 Bing Li 1 , Kaiyuan Wang 2 , Rui Zhang 1 , Baihui Li 1 , Yangli Shen 1 , Qinggang Ji 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-08-31 , DOI: 10.1016/j.ejmech.2019.111669 Bing Li 1 , Kaiyuan Wang 2 , Rui Zhang 1 , Baihui Li 1 , Yangli Shen 1 , Qinggang Ji 1
Affiliation
|
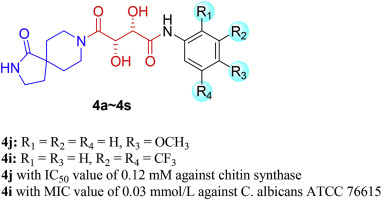
A series of 2,8-diazaspiro[4.5]decan-1-one derivatives were designed, synthesized and screened for their inhibition activities against chitin synthase (CHS) and antimicrobial activities in vitro. The biological assays revealed that compounds 4a, 4e, 4h, 4j, 4o, 4q and 4r exhibited moderated to excellent potency against CHS with IC50 values ranging from 0.12 to 0.29 mM. Compounds 4e, 4j with IC50 value of 0.13 mM, 0.12 mM respectively, showed excellent inhibition potency among these compounds, which were similar to that of polyoxin B whose IC50 value was 0.08 mM. Meanwhile, the screening of the antifungal activity showed that compounds 4j and 4r had the same potency of inhibiting the growth of A. fumigatus with MIC value of 0.08 mmol/L. Compound 4d displayed excellent activity against C. albicans (ATCC 90023) with MIC value of 0.04 mmol/L, which was superior to fluconazole (0.104 mmol/L) and polyoxin B (0.129 mmol/L). The result of antibacterial assay showed that these compounds had little potency against those selected bacteria strains including three Gram-positive bacteria and three Gram-negative bacteria. Furthermore, the combination use of 4c-fluconazole, 4i-fluconazole, 4j-fluconazole, and 4o-fluconazole against C. albicans,A. fumigatus and A. flavus showed additive or synergistic effects. These results indicated that the designed compounds serve as potential chitin synthase inhibitors and have selectively antifungal activities.
中文翻译:

新型二氮杂螺[4.5] decan-1-one衍生物作为潜在的几丁质合酶抑制剂和抗真菌剂的设计,合成和生物学评估。
设计,合成和筛选了一系列的2,8-二氮杂螺[4.5] decan-1-one衍生物对几丁质合酶(CHS)的抑制活性和体外抗菌活性。生物学分析表明,化合物4a,4e,4h,4j,4o,4q和4r表现出对CHS的中度至极强效价,IC50值为0.12至0.29 mM。化合物4e,4j的IC50值分别为0.13 mM和0.12 mM,在这些化合物中显示出优异的抑制能力,类似于多恶心素B的IC50值为0.08 mM。同时,抗真菌活性的筛选表明化合物4j和4r具有相同的抑制烟曲霉生长的效力,MIC值为0.08mmol / L。化合物4d对白色念珠菌(ATCC 90023)表现出优异的活性,MIC值为0.04 mmol / L,优于氟康唑(0.104 mmol / L)和多恶菌素B(0.129 mmol / L)。抗菌测定的结果表明,这些化合物对包括3种革兰氏阳性菌和3种革兰氏阴性菌在内的选定菌株没有什么作用。此外,将4c-氟康唑,4i-氟康唑,4j-氟康唑和4o-氟康唑联合使用以对抗白色念珠菌。烟熏和黄曲霉显示出加和或协同作用。这些结果表明,所设计的化合物可用作潜在的几丁质合酶抑制剂,并具有选择性的抗真菌活性。抗菌测定的结果表明,这些化合物对包括3种革兰氏阳性细菌和3种革兰氏阴性细菌在内的选定细菌菌株几乎没有效力。此外,将4c-氟康唑,4i-氟康唑,4j-氟康唑和4o-氟康唑联合使用以对抗白色念珠菌。烟熏和黄曲霉显示出加和或协同作用。这些结果表明,所设计的化合物可用作潜在的几丁质合酶抑制剂,并具有选择性的抗真菌活性。抗菌测定的结果表明,这些化合物对包括3种革兰氏阳性菌和3种革兰氏阴性菌在内的选定菌株没有什么作用。此外,将4c-氟康唑,4i-氟康唑,4j-氟康唑和4o-氟康唑联合使用以对抗白色念珠菌。烟熏和黄曲霉显示出加和或协同作用。这些结果表明,所设计的化合物可用作潜在的几丁质合酶抑制剂,并具有选择性的抗真菌活性。
更新日期:2019-08-31
中文翻译:

新型二氮杂螺[4.5] decan-1-one衍生物作为潜在的几丁质合酶抑制剂和抗真菌剂的设计,合成和生物学评估。
设计,合成和筛选了一系列的2,8-二氮杂螺[4.5] decan-1-one衍生物对几丁质合酶(CHS)的抑制活性和体外抗菌活性。生物学分析表明,化合物4a,4e,4h,4j,4o,4q和4r表现出对CHS的中度至极强效价,IC50值为0.12至0.29 mM。化合物4e,4j的IC50值分别为0.13 mM和0.12 mM,在这些化合物中显示出优异的抑制能力,类似于多恶心素B的IC50值为0.08 mM。同时,抗真菌活性的筛选表明化合物4j和4r具有相同的抑制烟曲霉生长的效力,MIC值为0.08mmol / L。化合物4d对白色念珠菌(ATCC 90023)表现出优异的活性,MIC值为0.04 mmol / L,优于氟康唑(0.104 mmol / L)和多恶菌素B(0.129 mmol / L)。抗菌测定的结果表明,这些化合物对包括3种革兰氏阳性菌和3种革兰氏阴性菌在内的选定菌株没有什么作用。此外,将4c-氟康唑,4i-氟康唑,4j-氟康唑和4o-氟康唑联合使用以对抗白色念珠菌。烟熏和黄曲霉显示出加和或协同作用。这些结果表明,所设计的化合物可用作潜在的几丁质合酶抑制剂,并具有选择性的抗真菌活性。抗菌测定的结果表明,这些化合物对包括3种革兰氏阳性细菌和3种革兰氏阴性细菌在内的选定细菌菌株几乎没有效力。此外,将4c-氟康唑,4i-氟康唑,4j-氟康唑和4o-氟康唑联合使用以对抗白色念珠菌。烟熏和黄曲霉显示出加和或协同作用。这些结果表明,所设计的化合物可用作潜在的几丁质合酶抑制剂,并具有选择性的抗真菌活性。抗菌测定的结果表明,这些化合物对包括3种革兰氏阳性菌和3种革兰氏阴性菌在内的选定菌株没有什么作用。此外,将4c-氟康唑,4i-氟康唑,4j-氟康唑和4o-氟康唑联合使用以对抗白色念珠菌。烟熏和黄曲霉显示出加和或协同作用。这些结果表明,所设计的化合物可用作潜在的几丁质合酶抑制剂,并具有选择性的抗真菌活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号