当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification and characterization of benzo[d]oxazol-2(3H)-one derivatives as the first potent and selective small-molecule inhibitors of chromodomain protein CDYL.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-08-31 , DOI: 10.1016/j.ejmech.2019.111656 Lixin Yang 1 , Yongqing Liu 1 , Minghua Fan 1 , Guiwang Zhu 1 , Hongwei Jin 1 , Jing Liang 2 , Zhenming Liu 1 , Zhuo Huang 1 , Liangren Zhang 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-08-31 , DOI: 10.1016/j.ejmech.2019.111656 Lixin Yang 1 , Yongqing Liu 1 , Minghua Fan 1 , Guiwang Zhu 1 , Hongwei Jin 1 , Jing Liang 2 , Zhenming Liu 1 , Zhuo Huang 1 , Liangren Zhang 1
Affiliation
|
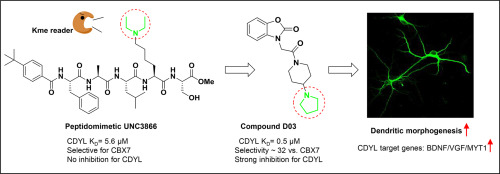
Chemical probes of epigenetic 'readers' of histone post-translational modifications (PTMs) have become powerful tools for mechanistic and functional studies of their target proteins in physiology and pathology. However, only limited 'reader' probes have been developed, which restricted our understanding towards these macromolecules and their roles in cells or animals. Here, we reported a structure-guided approach to develop and characterize benzo [d]oxazol-2(3H)-one analogs as the first potent and selective small-molecule inhibitors of chromodomain Y-like (CDYL), a histone methyllysine reader protein. The binding conformation between the chromodomain of CDYL and the modified peptidomimetics was studied via molecular docking and dynamic simulations, facilitating subsequent virtual screening of tens of hits from Specs chemical library validated by SPR technique (KD values: from 271.1 μM to 5.4 μM). Further design and synthesis of 43 compounds helped to interpret the structure-activity relationship (SAR) that lead to the discovery of novel small-molecule inhibitors of CDYL. Compound D03 (KD: 0.5 μM) was discovered and showed excellent selectivity among other chromodomain proteins, including CDYL2 (>140 folds), CDY1 (no observed binding) and CBX7 (>32 folds). Moreover, we demonstrated that D03 engaged with endogenous CDYL in a dose-dependent manner, and perturbed the recruitment of CDYL onto chromatin, resulting in transcriptional derepression of its target genes. Finally, the results showed that D03 promoted the development and branching of neurodendrites by inhibiting CDYL in hippocampal and cortical cultured neurons. This study not only discovers the first selective small-molecule inhibitors of CDYL, but provids a new chemical tool to intervene the dynamic nature of bio-macromolecules involved in epigenetic mechanism.
中文翻译:

苯并[d]恶唑-2(3H)-one衍生物作为色域蛋白CDYL的第一个有效和选择性小分子抑制剂的鉴定和表征。
组蛋白翻译后修饰(PTM)的表观遗传“阅读器”的化学探针已成为在生理学和病理学上对其靶蛋白进行机理和功能研究的强大工具。然而,仅开发了有限的“阅读器”探针,这限制了我们对这些大分子及其在细胞或动物中的作用的理解。在这里,我们报道了一种结构导向的方法来开发和表征苯并[d]恶唑-2(3H)-类似物,作为第一个有效的和选择性的小分子抑制剂,称为色域Y样(CDYL),一种组蛋白甲基赖氨酸阅读器蛋白。通过分子对接和动态模拟研究了CDYL的色域与修饰的拟肽之间的结合构象,便于随后从Specs化学文库中通过SPR技术验证的数十个命中点进行虚拟筛选(KD值:从271.1μM到5.4μM)。43种化合物的进一步设计和合成有助于解释结构-活性关系(SAR),这导致发现了新型CDYL小分子抑制剂。发现了化合物D03(KD:0.5μM),并且在其他色域蛋白中表现出优异的选择性,包括CDYL2(> 140倍),CDY1(未观察到结合)和CBX7(> 32倍)。此外,我们证明D03与内源性CDYL呈剂量依赖性,并干扰CDYL在染色质上的募集,从而导致其靶基因的转录抑制。最后,结果表明,D03通过抑制海马和皮层培养的神经元中的CDYL,促进神经树突的发育和分支。这项研究不仅发现了第一个选择性的CDYL小分子抑制剂,而且提供了一种新的化学工具来干预参与表观遗传机制的生物大分子的动态性质。
更新日期:2019-08-31
中文翻译:

苯并[d]恶唑-2(3H)-one衍生物作为色域蛋白CDYL的第一个有效和选择性小分子抑制剂的鉴定和表征。
组蛋白翻译后修饰(PTM)的表观遗传“阅读器”的化学探针已成为在生理学和病理学上对其靶蛋白进行机理和功能研究的强大工具。然而,仅开发了有限的“阅读器”探针,这限制了我们对这些大分子及其在细胞或动物中的作用的理解。在这里,我们报道了一种结构导向的方法来开发和表征苯并[d]恶唑-2(3H)-类似物,作为第一个有效的和选择性的小分子抑制剂,称为色域Y样(CDYL),一种组蛋白甲基赖氨酸阅读器蛋白。通过分子对接和动态模拟研究了CDYL的色域与修饰的拟肽之间的结合构象,便于随后从Specs化学文库中通过SPR技术验证的数十个命中点进行虚拟筛选(KD值:从271.1μM到5.4μM)。43种化合物的进一步设计和合成有助于解释结构-活性关系(SAR),这导致发现了新型CDYL小分子抑制剂。发现了化合物D03(KD:0.5μM),并且在其他色域蛋白中表现出优异的选择性,包括CDYL2(> 140倍),CDY1(未观察到结合)和CBX7(> 32倍)。此外,我们证明D03与内源性CDYL呈剂量依赖性,并干扰CDYL在染色质上的募集,从而导致其靶基因的转录抑制。最后,结果表明,D03通过抑制海马和皮层培养的神经元中的CDYL,促进神经树突的发育和分支。这项研究不仅发现了第一个选择性的CDYL小分子抑制剂,而且提供了一种新的化学工具来干预参与表观遗传机制的生物大分子的动态性质。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号