当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Carbon Isotope Fractionation of 1,2-Dibromoethane by Biological and Abiotic Processes
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.est.7b05224 Paul G. Koster van Groos 1 , Paul B. Hatzinger 1 , Sheryl H. Streger 1 , Simon Vainberg 1 , R. Paul Philp 2 , Tomasz Kuder 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2018-03-01 00:00:00 , DOI: 10.1021/acs.est.7b05224 Paul G. Koster van Groos 1 , Paul B. Hatzinger 1 , Sheryl H. Streger 1 , Simon Vainberg 1 , R. Paul Philp 2 , Tomasz Kuder 2
Affiliation
|
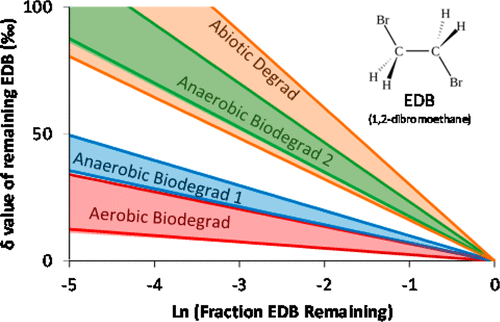
1,2-Dibromethane (EDB) is a toxic fuel additive that likely occurs at many sites where leaded fuels have impacted groundwater. This study quantified carbon (C) isotope fractionation of EDB associated with anaerobic and aerobic biodegradation, abiotic degradation by iron sulfides, and abiotic hydrolysis. These processes likely contribute to EDB degradation in source zones (biodegradation) and in more dilute plumes (hydrolysis). Mixed anaerobic cultures containing dehalogenating organisms (e.g., Dehaloccoides spp.) were examined, as were aerobic cultures that degrade EDB cometabolically. Bulk C isotope enrichment factors (εbulk) associated with biological degradation covered a large range, with mixed anaerobic cultures fractionating more (εbulk from −8 to −20‰) than aerobic cultures (εbulk from −3 to −6‰). εbulk magnitudes associated with the abiotic processes (dihaloelimination by FeS/FeS2 and hydrolysis) were large but fairly well constrained (εbulk from −19 to −29‰). As expected, oxidative mechanisms fractionated EDB less than dihaloelimination and substitution mechanisms, and biological systems exhibited a larger range of fractionation, potentially due to isotope masking effects. In addition to quantifying and discussing εbulk values, which are highly relevant for quantifying in situ EDB degradation, an innovative approach for constraining the age of EDB in the aqueous phase, based on fractionation during hydrolysis, is described.
中文翻译:

生物和非生物过程分离1,2-二溴乙烷的碳同位素
1,2-二溴甲烷(EDB)是一种有毒的燃料添加剂,可能发生在含铅燃料影响地下水的许多地点。这项研究量化了EDB的碳(C)同位素分级,该分级与厌氧和好氧生物降解,硫化铁的非生物降解以及非生物水解有关。这些过程可能会导致EDB在源区降解(生物降解)和更稀薄的羽状流(水解)。检查了包含脱卤生物(例如Dehaloccoides spp。)的混合厌氧培养物,以及可代谢分解EDB的需氧培养物。大块c-同位素富集因子(ε散装与生物降解有关)覆盖的大范围内,混合厌氧培养物分馏更多(ε散装从-8到-20‰)比有氧培养(ε体积从-3到-6‰)。与非生物过程(FeS / FeS 2的二卤代消除和水解作用)相关的ε体积大小很大,但受到很好的限制(ε体积从-19到-29‰)。不出所料,氧化机制对EDB的分馏作用少于二卤代消除和取代机制,而生物系统则表现出更大范围的分馏作用,这可能是由于同位素掩蔽作用所致。除了量化和讨论与定量EDB降解高度相关的ε体积值外,还描述了一种基于水解过程中的分馏来限制水相中EDB年龄的创新方法。
更新日期:2018-03-01
中文翻译:

生物和非生物过程分离1,2-二溴乙烷的碳同位素
1,2-二溴甲烷(EDB)是一种有毒的燃料添加剂,可能发生在含铅燃料影响地下水的许多地点。这项研究量化了EDB的碳(C)同位素分级,该分级与厌氧和好氧生物降解,硫化铁的非生物降解以及非生物水解有关。这些过程可能会导致EDB在源区降解(生物降解)和更稀薄的羽状流(水解)。检查了包含脱卤生物(例如Dehaloccoides spp。)的混合厌氧培养物,以及可代谢分解EDB的需氧培养物。大块c-同位素富集因子(ε散装与生物降解有关)覆盖的大范围内,混合厌氧培养物分馏更多(ε散装从-8到-20‰)比有氧培养(ε体积从-3到-6‰)。与非生物过程(FeS / FeS 2的二卤代消除和水解作用)相关的ε体积大小很大,但受到很好的限制(ε体积从-19到-29‰)。不出所料,氧化机制对EDB的分馏作用少于二卤代消除和取代机制,而生物系统则表现出更大范围的分馏作用,这可能是由于同位素掩蔽作用所致。除了量化和讨论与定量EDB降解高度相关的ε体积值外,还描述了一种基于水解过程中的分馏来限制水相中EDB年龄的创新方法。















































 京公网安备 11010802027423号
京公网安备 11010802027423号