当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crown Ether Effects on the Location of Charge Carriers in Electrospray Droplets: Implications for the Mechanism of Protein Charging and Supercharging
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.analchem.8b00099 Haidy Metwally 1 , Lars Konermann 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-02-28 00:00:00 , DOI: 10.1021/acs.analchem.8b00099 Haidy Metwally 1 , Lars Konermann 1
Affiliation
|
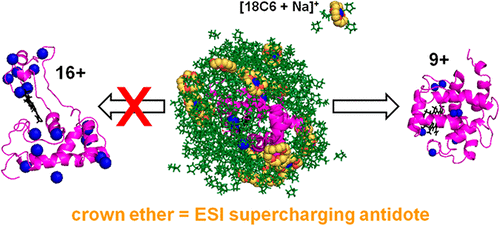
“Native” electrospray ionization (ESI) mass spectrometry (MS) aims to transfer proteins from solution into the gas phase while maintaining solution-like structures and interactions. The ability to control the charge states of protein ions produced in these experiments is of considerable importance. Supercharging agents (SCAs) such as sulfolane greatly elevate charge states without significantly affecting the protein structure in bulk aqueous solution. The origin of native ESI supercharging remains contentious. According to one model, SCAs trigger unfolding within ESI droplets. In contrast, the “charge trapping model” envisions that SCAs impede the ejection of charge carriers (e.g., NH4+ or Na+) from the droplet. We addressed this controversy experimentally and computationally by employing 18C6 crown ether as a mechanistic probe in native ESI-MS experiments on holo-myoglobin. Remarkably, 18C6 suppressed the supercharging capability of sulfolane. Molecular dynamics (MD) simulations reproduced the experimental charge states. The MD data revealed that 18C6 altered the location of charge carriers in the ESI droplets. Without 18C6, sulfolane covered the droplets in an ionophobic layer that impeded charge carrier access to the surface. In contrast, 18C6 complexation caused charge carrier enrichment in this surface layer, thereby promoting charge ejection. For late droplets, all the water had left and the protein was encapsulated in sulfolane; charge ejection at this stage continued only in the presence of 18C6. As a result, evaporation to dryness of charge-depleted water/sulfolane/18C6 droplets produced low protein charge states, whereas charge-abundant water/sulfolane droplets generated high charge states. Our data support the view that native ESI supercharging is caused by charge trapping. Unfolding within the droplet may play an ancillary role under some conditions, but for the cases examined here, protein structural changes are not a causative factor for supercharging. Our conclusions are bolstered by dendrimer supercharging experiments.
中文翻译:

冠醚对电喷雾液滴中电荷载体位置的影响:对蛋白质充电和增压机制的影响
“天然”电喷雾电离(ESI)质谱(MS)旨在将蛋白质从溶液转移到气相中,同时保持类似溶液的结构和相互作用。控制在这些实验中产生的蛋白质离子的电荷状态的能力非常重要。诸如环丁砜的增压剂(SCA)可以大大提高电荷状态,而不会显着影响本体水溶液中的蛋白质结构。本地ESI增压的起源仍然存在争议。根据一种模型,SCA触发ESI小滴内的展开。相反,“电荷俘获模型”设想SCA会阻止电荷载流子(例如NH 4 +或Na +)。我们通过在天然肌红蛋白的天然ESI-MS实验中采用18C6冠醚作为机械探针,以实验和计算方式解决了这一争议。值得注意的是,18 C 6抑制了环丁砜的增压能力。分子动力学(MD)模拟重现了实验电荷状态。MD数据显示18C6改变了ESI液滴中电荷载流子的位置。在没有18C6的情况下,环丁砜会覆盖疏液层中的液滴,从而阻止电荷载流子进入表面。相反,18 C 6络合导致该表面层中的载流子富集,从而促进电荷喷射。对于较晚的飞沫,所有的水都已剩下,蛋白质被包裹在环丁砜中;仅在18C6存在的情况下,该阶段的电荷喷射才继续进行。因此,电荷耗尽的水/环丁砜/ 18C6液滴蒸发至干时产生的蛋白质电荷状态低,而电荷丰富的水/环丁砜液滴产生较高的电荷状态。我们的数据支持以下观点:原生ESI增压是由电荷陷阱引起的。在某些情况下,液滴内的展开可能起辅助作用,但对于此处检查的情况,蛋白质结构变化不是增压的诱因。树枝状大分子增压实验支持了我们的结论。但对于此处检查的情况,蛋白质结构的变化并不是增压的诱因。树枝状大分子增压实验支持了我们的结论。但对于此处检查的情况,蛋白质结构的变化并不是增压的诱因。树枝状大分子增压实验支持了我们的结论。
更新日期:2018-02-28
中文翻译:

冠醚对电喷雾液滴中电荷载体位置的影响:对蛋白质充电和增压机制的影响
“天然”电喷雾电离(ESI)质谱(MS)旨在将蛋白质从溶液转移到气相中,同时保持类似溶液的结构和相互作用。控制在这些实验中产生的蛋白质离子的电荷状态的能力非常重要。诸如环丁砜的增压剂(SCA)可以大大提高电荷状态,而不会显着影响本体水溶液中的蛋白质结构。本地ESI增压的起源仍然存在争议。根据一种模型,SCA触发ESI小滴内的展开。相反,“电荷俘获模型”设想SCA会阻止电荷载流子(例如NH 4 +或Na +)。我们通过在天然肌红蛋白的天然ESI-MS实验中采用18C6冠醚作为机械探针,以实验和计算方式解决了这一争议。值得注意的是,18 C 6抑制了环丁砜的增压能力。分子动力学(MD)模拟重现了实验电荷状态。MD数据显示18C6改变了ESI液滴中电荷载流子的位置。在没有18C6的情况下,环丁砜会覆盖疏液层中的液滴,从而阻止电荷载流子进入表面。相反,18 C 6络合导致该表面层中的载流子富集,从而促进电荷喷射。对于较晚的飞沫,所有的水都已剩下,蛋白质被包裹在环丁砜中;仅在18C6存在的情况下,该阶段的电荷喷射才继续进行。因此,电荷耗尽的水/环丁砜/ 18C6液滴蒸发至干时产生的蛋白质电荷状态低,而电荷丰富的水/环丁砜液滴产生较高的电荷状态。我们的数据支持以下观点:原生ESI增压是由电荷陷阱引起的。在某些情况下,液滴内的展开可能起辅助作用,但对于此处检查的情况,蛋白质结构变化不是增压的诱因。树枝状大分子增压实验支持了我们的结论。但对于此处检查的情况,蛋白质结构的变化并不是增压的诱因。树枝状大分子增压实验支持了我们的结论。但对于此处检查的情况,蛋白质结构的变化并不是增压的诱因。树枝状大分子增压实验支持了我们的结论。































 京公网安备 11010802027423号
京公网安备 11010802027423号