当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Color Switching in Dual-Emitting Mn(II)-Doped CsPbBr3 Perovskite Nanorods: Dilution versus Evaporation
ACS Energy Letters ( IF 19.3 ) Pub Date : 2019-09-09 , DOI: 10.1021/acsenergylett.9b01702 Shyamal Kumar Mehetor 1 , Harekrishna Ghosh 1 , Biswajit Hudait 1 , Niladri Sekhar Karan 2 , Animesh Paul 3 , Sujoy Baitalik 3 , Narayan Pradhan 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2019-09-09 , DOI: 10.1021/acsenergylett.9b01702 Shyamal Kumar Mehetor 1 , Harekrishna Ghosh 1 , Biswajit Hudait 1 , Niladri Sekhar Karan 2 , Animesh Paul 3 , Sujoy Baitalik 3 , Narayan Pradhan 1
Affiliation
|
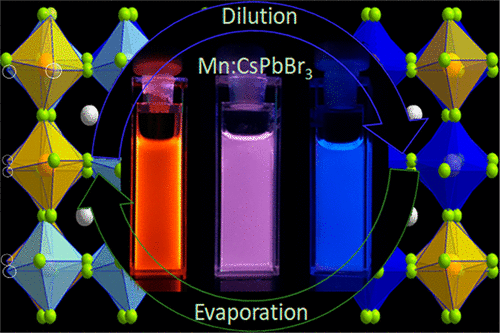
Postsynthesis halide treatment can brighten perovskite nanocrystals. While this has been recently explored for undoped nanocrystals having only the exciton emission, herein, the impact of halide-enriched and -deficient environments was studied for dual-emitting Mn(II)-doped CsPbBr3 nanorods. This was performed by adopting a self-regulated approach where the nanorod solution reversibly switched the color brightness from blue to orange and vice versa by dilution and evaporation, respectively. With control experiments, it was established that the color switching was not due to a change in the rate of the exciton energy transfer from host to dopant energy states; rather, it was related to self-regulated fulfilling and again creating halide vacancies observed with variation of nanorod concentration. Being that the halide vacancy was established as one of the key factors for controlling the brightness of these nanocrystals, this reversible switching in doped CsPbBr3 adds new fundamental insight into controlling the photoluminescence of these emerging nanocrystals.
中文翻译:

掺锰(II)的CsPbBr3钙钛矿纳米棒中的可逆颜色切换:稀释与蒸发
合成后的卤化物处理可以使钙钛矿纳米晶体变亮。尽管最近已对仅具有激子发射的未掺杂纳米晶体进行了探索,但在此,针对双发射掺杂Mn(II)的CsPbBr 3研究了卤化物富集和不足的环境的影响。纳米棒。这是通过采用自我调节的方法完成的,其中纳米棒溶液分别通过稀释和蒸发可逆地将颜色亮度从蓝色切换为橙色,反之亦然。通过对照实验,可以确定颜色切换不是由于激子能量从主体到掺杂能态的转移速率的变化所致。相反,它与自我调节的满足感有关,并且随着纳米棒浓度的变化而再次产生卤化物空位。由于已将卤化物空位确定为控制这些纳米晶体亮度的关键因素之一,因此掺杂CsPbBr 3的这种可逆转换为控制这些新兴纳米晶体的光致发光提供了新的基础知识。
更新日期:2019-09-09
中文翻译:

掺锰(II)的CsPbBr3钙钛矿纳米棒中的可逆颜色切换:稀释与蒸发
合成后的卤化物处理可以使钙钛矿纳米晶体变亮。尽管最近已对仅具有激子发射的未掺杂纳米晶体进行了探索,但在此,针对双发射掺杂Mn(II)的CsPbBr 3研究了卤化物富集和不足的环境的影响。纳米棒。这是通过采用自我调节的方法完成的,其中纳米棒溶液分别通过稀释和蒸发可逆地将颜色亮度从蓝色切换为橙色,反之亦然。通过对照实验,可以确定颜色切换不是由于激子能量从主体到掺杂能态的转移速率的变化所致。相反,它与自我调节的满足感有关,并且随着纳米棒浓度的变化而再次产生卤化物空位。由于已将卤化物空位确定为控制这些纳米晶体亮度的关键因素之一,因此掺杂CsPbBr 3的这种可逆转换为控制这些新兴纳米晶体的光致发光提供了新的基础知识。































 京公网安备 11010802027423号
京公网安备 11010802027423号