当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ylide-Functionalized Phosphine (YPhos)-Palladium Catalysts: Selective Monoarylation of Alkyl Ketones with Aryl Chlorides.
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-30 , DOI: 10.1021/acs.orglett.9b02830 Xiao-Qiang Hu 1 , Dominik Lichte 1 , Ilja Rodstein 2 , Philip Weber 1 , Ann-Katrin Seitz 1 , Thorsten Scherpf 2 , Viktoria H Gessner 2 , Lukas J Gooßen 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-30 , DOI: 10.1021/acs.orglett.9b02830 Xiao-Qiang Hu 1 , Dominik Lichte 1 , Ilja Rodstein 2 , Philip Weber 1 , Ann-Katrin Seitz 1 , Thorsten Scherpf 2 , Viktoria H Gessner 2 , Lukas J Gooßen 1
Affiliation
|
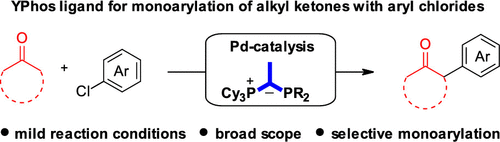
Ylide-functionalized phosphine (YPhos) ligands allow the palladium-catalyzed α-arylation of alkyl ketones with aryl chlorides with record setting activity. Using a cyclohexyl-substituted YPhos ligand, a wide range of challenging ketone substrates was efficiently and selectively monoarylated under mild conditions. A newly designed YPhos ligand bearing tert-butyl groups on the coordinating phosphorus atom is already active at room temperature. The synthetic potential was demonstrated by gram-scale reactions and the succinct synthesis of ε-caprolactone derivatives.
中文翻译:

Ylide功能化的膦(YPhos)-钯催化剂:烷基酮与芳基氯化物的选择性单芳基化。
内酯官能化的膦(YPhos)配体允许钯催化烷基酮与芳基氯化物的α-芳基化,具有创纪录的活性。使用环己基取代的YPhos配体,可在温和条件下有效且选择性地对各种具有挑战性的酮底物进行有效且选择性的单芳基化。一种新设计的在配位磷原子上带有叔丁基的YPhos配体已在室温下活跃。通过克规模反应和ε-己内酯衍生物的简洁合成证明了合成潜力。
更新日期:2019-08-31
中文翻译:

Ylide功能化的膦(YPhos)-钯催化剂:烷基酮与芳基氯化物的选择性单芳基化。
内酯官能化的膦(YPhos)配体允许钯催化烷基酮与芳基氯化物的α-芳基化,具有创纪录的活性。使用环己基取代的YPhos配体,可在温和条件下有效且选择性地对各种具有挑战性的酮底物进行有效且选择性的单芳基化。一种新设计的在配位磷原子上带有叔丁基的YPhos配体已在室温下活跃。通过克规模反应和ε-己内酯衍生物的简洁合成证明了合成潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号