当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient synthesis of novel cyclic fused-phenothiazines via domino cyclization of 2-(benzo[b][1,4]thiazin-3-ylidene)acetate, aromatic aldehydes and cyclic 1,3-diketones†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019-08-30 , DOI: 10.1039/c9qo00951e Jing Sun 1, 2, 3, 4 , Quan-Shun Sun 1, 2, 3, 4 , Chao-Guo Yan 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019-08-30 , DOI: 10.1039/c9qo00951e Jing Sun 1, 2, 3, 4 , Quan-Shun Sun 1, 2, 3, 4 , Chao-Guo Yan 1, 2, 3, 4
Affiliation
|
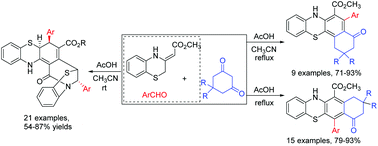
Functionalized 2,3,4,7-tetrahydrobenzo[c]phenothiazines and 7,9,10,12-tetrahydrobenzo[b]phenothiazines were selectively synthesized from a three-component reaction of aromatic aldehydes, cyclic 1,3-diketones and methyl 2-(benzo[b][1,4]thiazin-3-ylidene)acetate in refluxing AcOH/CH3CN medium or in refluxing acetic acid. On the other hand, the domino cyclization reaction of aromatic aldehydes with methyl 2-(benzo[b][1,4]thiazin-3-ylidene)acetate in AcOH/CH3CN medium resulted in polycyclic 8,14-methanobenzo[2,3][1,4]thiazocino[6,7-a]phenothiazines in satisfactory yields. Additionally, similar reactions with some typical 1,2-dicarbonyl compounds afforded diverse polycyclic compounds.
中文翻译:

新型环状稠吩噻嗪的有效合成通过2-(苯并[多米诺环化b ] [1,4]噻嗪-3-亚基)乙酸乙酯,芳香醛和环状1,3-二酮†
从芳族醛,环状1,3-二酮和甲基2的三组分反应中选择性合成功能化的2,3,4,7-四氢苯并[ c ]吩噻嗪和7,9,10,12-四氢苯并[ b ]吩噻嗪在回流的AcOH / CH 3 CN介质中或在回流的乙酸中生成-(苯并[ b ] [1,4]噻嗪-3-亚丙基)乙酸酯。另一方面,芳香醛与2-(苯并[ b ] [1,4]噻嗪-3-亚烷基)乙酸甲酯在AcOH / CH 3 CN介质中的多米诺环化反应生成多环8,14-甲氧基苯并[2] ,3] [1,4] thiazocino [6,7- a]吩噻嗪,收率令人满意。另外,与一些典型的1,2-二羰基化合物的类似反应提供了多种多环化合物。
更新日期:2019-10-10
中文翻译:

新型环状稠吩噻嗪的有效合成通过2-(苯并[多米诺环化b ] [1,4]噻嗪-3-亚基)乙酸乙酯,芳香醛和环状1,3-二酮†
从芳族醛,环状1,3-二酮和甲基2的三组分反应中选择性合成功能化的2,3,4,7-四氢苯并[ c ]吩噻嗪和7,9,10,12-四氢苯并[ b ]吩噻嗪在回流的AcOH / CH 3 CN介质中或在回流的乙酸中生成-(苯并[ b ] [1,4]噻嗪-3-亚丙基)乙酸酯。另一方面,芳香醛与2-(苯并[ b ] [1,4]噻嗪-3-亚烷基)乙酸甲酯在AcOH / CH 3 CN介质中的多米诺环化反应生成多环8,14-甲氧基苯并[2] ,3] [1,4] thiazocino [6,7- a]吩噻嗪,收率令人满意。另外,与一些典型的1,2-二羰基化合物的类似反应提供了多种多环化合物。















































 京公网安备 11010802027423号
京公网安备 11010802027423号