当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of 1-benzyl-5-oxopyrrolidine-2-carboximidamide derivatives as novel neuroprotective agents.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-08-28 , DOI: 10.1016/j.ejmech.2019.111654 Linkui Zhang 1 , Jishun Quan 1 , Ying Zhao 1 , Donglin Yang 1 , Qingchun Zhao 2 , Peng Liu 1 , Maosheng Cheng 1 , Chao Ma 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-08-28 , DOI: 10.1016/j.ejmech.2019.111654 Linkui Zhang 1 , Jishun Quan 1 , Ying Zhao 1 , Donglin Yang 1 , Qingchun Zhao 2 , Peng Liu 1 , Maosheng Cheng 1 , Chao Ma 1
Affiliation
|
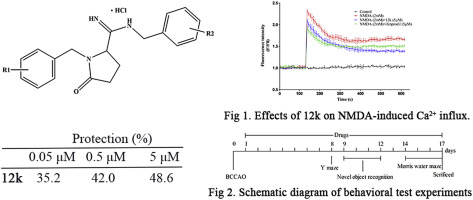
A series of 1-benzyl-5-oxopyrrolidine-2-carboximidamide derivatives were designed and synthesized. Their protective activities against N-methyl-d-aspartic acid (NMDA)-induced cytotoxicity were investigated in vitro. All of the compounds exhibited neuroprotective activities, especially 12k, which showed higher potency than reference compound 1 (ifenprodil). Further investigation showed that 12k could attenuate Ca2+ influx and suppress the NR2B upregulation induced by NMDA. The docking results indicated that 12k could fit well into binding site of 1 in the NR2B-NMDA receptor. Additionally, 12k exhibited excellent metabolic stability. Furthermore, the results of behavioral tests showed that compound 12k could significantly improve learning and memory in vivo. These results suggested that 12k is a promising neuroprotective drug candidate and that the NR2B-NMDA receptor is a potential target of 12k.
中文翻译:

作为新型神经保护剂的1-苄基5-氧吡咯烷-2-羧酰亚胺酰胺衍生物的设计,合成和生物学评估。
设计并合成了一系列的1-苄基-5-氧吡咯烷-2-羧酰亚胺酰胺衍生物。在体外研究了它们对N-甲基-d-天冬氨酸(NMDA)诱导的细胞毒性的保护活性。所有化合物均表现出神经保护活性,尤其是12k,其显示出比参考化合物1(艾芬地尔)更高的效力。进一步的研究表明12k可以减弱Ca2 +的流入并抑制NMDA诱导的NR2B上调。对接结果表明12k可以很好地适合NR2B-NMDA受体1的结合位点。另外,12k表现出优异的代谢稳定性。此外,行为测试的结果表明,化合物12k可以显着改善体内的学习和记忆能力。
更新日期:2019-08-28
中文翻译:

作为新型神经保护剂的1-苄基5-氧吡咯烷-2-羧酰亚胺酰胺衍生物的设计,合成和生物学评估。
设计并合成了一系列的1-苄基-5-氧吡咯烷-2-羧酰亚胺酰胺衍生物。在体外研究了它们对N-甲基-d-天冬氨酸(NMDA)诱导的细胞毒性的保护活性。所有化合物均表现出神经保护活性,尤其是12k,其显示出比参考化合物1(艾芬地尔)更高的效力。进一步的研究表明12k可以减弱Ca2 +的流入并抑制NMDA诱导的NR2B上调。对接结果表明12k可以很好地适合NR2B-NMDA受体1的结合位点。另外,12k表现出优异的代谢稳定性。此外,行为测试的结果表明,化合物12k可以显着改善体内的学习和记忆能力。










































 京公网安备 11010802027423号
京公网安备 11010802027423号