当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,2-Boron Shifts of β-Boryl Radicals Generated from Bis-Boronic Esters using Photoredox Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-08-28 , DOI: 10.1021/jacs.9b07564 Daniel Kaiser 1 , Adam Noble 1 , Valerio Fasano 1 , Varinder K Aggarwal 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-08-28 , DOI: 10.1021/jacs.9b07564 Daniel Kaiser 1 , Adam Noble 1 , Valerio Fasano 1 , Varinder K Aggarwal 1
Affiliation
|
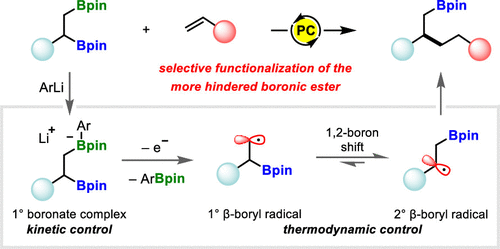
1,2-Bis-boronic esters are versatile intermediates that enable the rapid elaboration of simple alkene precursors. Previous reports on their selective mono-functionalization have targeted the most accessible position, retaining the more hindered secondary boronic ester. In contrast, we have found that photoredox-catalyzed mono-deboronation generates primary β-boryl radicals that undergo rapid 1,2-boron shift to form thermodynamically favored secondary radicals, allowing for selective transformation of the more hindered boronic ester. The pivotal 1,2-boron shift, which has been demonstrated to be stereoretentive, enables access to a wide range of functionalized boronic esters and has been applied to highly diastereoselective fragmentation and transannular cyclization reactions. Furthermore, its generality has been shown in a radical cascade reaction with an allylboronic ester.
中文翻译:

使用光氧化还原催化从双硼酸酯生成的 β-硼基自由基的 1,2-硼位移
1,2-双硼酸酯是多功能中间体,可以快速制备简单的烯烃前体。先前关于其选择性单官能化的报告针对的是最容易接近的位置,保留了受阻更多的仲硼酸酯。相比之下,我们发现光氧化还原催化的单脱硼作用会产生初级 β-硼基自由基,这些自由基经历快速的 1,2-硼转移以形成热力学有利的二级自由基,从而允许更多受阻硼酸酯的选择性转化。已被证明具有立体保留性的关键 1,2-硼位移能够获得广泛的官能化硼酸酯,并已应用于高度非对映选择性断裂和跨环环化反应。此外,
更新日期:2019-08-28
中文翻译:

使用光氧化还原催化从双硼酸酯生成的 β-硼基自由基的 1,2-硼位移
1,2-双硼酸酯是多功能中间体,可以快速制备简单的烯烃前体。先前关于其选择性单官能化的报告针对的是最容易接近的位置,保留了受阻更多的仲硼酸酯。相比之下,我们发现光氧化还原催化的单脱硼作用会产生初级 β-硼基自由基,这些自由基经历快速的 1,2-硼转移以形成热力学有利的二级自由基,从而允许更多受阻硼酸酯的选择性转化。已被证明具有立体保留性的关键 1,2-硼位移能够获得广泛的官能化硼酸酯,并已应用于高度非对映选择性断裂和跨环环化反应。此外,































 京公网安备 11010802027423号
京公网安备 11010802027423号