当前位置:
X-MOL 学术
›
Microchim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CdS quantum dots generated in-situ for fluorometric determination of thrombin activity
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-08-29 , DOI: 10.1007/s00604-019-3765-2 Laura Saa , Beatriz Díez-Buitrago , Nerea Briz , Valeri Pavlov
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-08-29 , DOI: 10.1007/s00604-019-3765-2 Laura Saa , Beatriz Díez-Buitrago , Nerea Briz , Valeri Pavlov
|
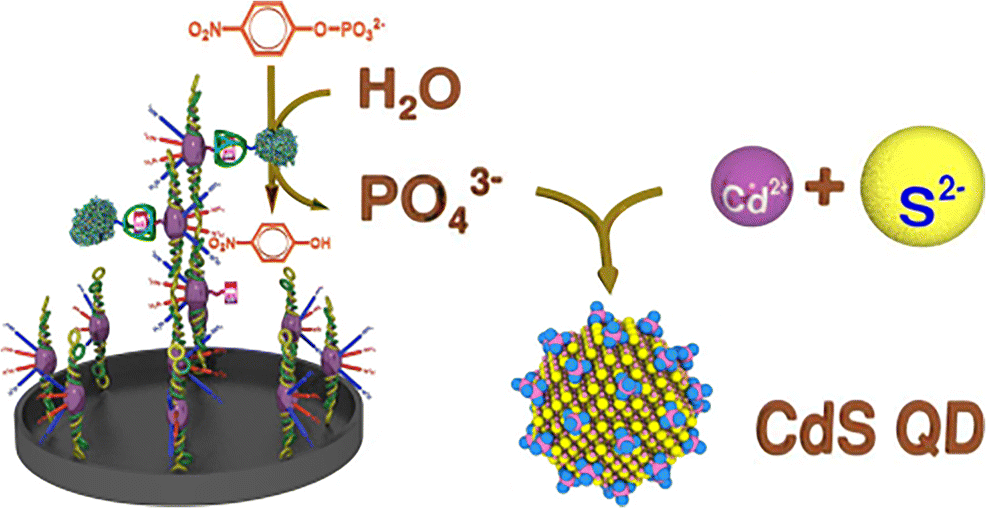
A method is presented for sensitive determination of thrombin activity. It is based on (a) the interaction between fibrinogen after activation with thrombin, and (b) an enzymatic amplification step consisting of in-situ growth of CdS quantum dots (QDs). Fibrinogen is immobilized on the surface of the wells of a microplate and then incubated with a mixture of biotinylated fibrinogen and thrombin. Thrombin activates immobilized fibrinogen and free biotinylated fibrinogen. This leads to the formation of insoluble biotinylated fibrin that remains bound on the surface of the wells. Afterwards, the samples are incubated with avidin-labeled alkaline phosphatase (ALP) which binds to biotinylated fibrin. ALP hydrolyzes the substrate p-nitrophenyl phosphate (pNPP) under formation of phosphate ions which stabilize CdS QDs that are grown in-situ from cadmium(II) and sulfide. The generation of fibrin is correlated with the activity of thrombin. Increased thrombin concentration results in increased fluorescence that can be measured at excitation/emission wavelengths of 300/510 nm. The introduction of such an amplification step (the enzyme-triggered growth of QDs) allows for the quantification of thrombin in the picomolar concentration range, with a linear response up to 2.5 pM and a detection limit of 0.05 pM. The method was applied to the determination of thrombin activity in human plasma and of the thrombin inhibitor argatroban. Graphical abstract Schematic representation of a fluorometric method for determination of thrombin activity in the picomolar concentration range based on the interaction between fibrinogen after activation with thrombin, and an enzymatic amplification step consisting of in-situ growth of CdS quantum dots (CdS QD). Schematic representation of a fluorometric method for determination of thrombin activity in the picomolar concentration range based on the interaction between fibrinogen after activation with thrombin, and an enzymatic amplification step consisting of in-situ growth of CdS quantum dots (CdS QD).
中文翻译:

原位生成的 CdS 量子点用于凝血酶活性的荧光测定
提出了一种灵敏测定凝血酶活性的方法。它基于 (a) 用凝血酶激活后纤维蛋白原之间的相互作用,以及 (b) 由 CdS 量子点 (QD) 原位生长组成的酶促放大步骤。纤维蛋白原固定在微孔板的孔表面,然后与生物素化纤维蛋白原和凝血酶的混合物一起孵育。凝血酶激活固定的纤维蛋白原和游离的生物素化纤维蛋白原。这导致形成不溶性生物素化纤维蛋白,其仍结合在孔表面。然后,将样品与与生物素化纤维蛋白结合的亲和素标记的碱性磷酸酶 (ALP) 一起孵育。ALP 水解底物对硝基苯基磷酸酯 (pNPP),形成磷酸根离子,稳定由镉 (II) 和硫化物原位生长的 CdS QD。纤维蛋白的产生与凝血酶的活性有关。凝血酶浓度增加导致荧光增加,可在 300/510 nm 的激发/发射波长下测量。这种扩增步骤(QD 的酶触发生长)的引入允许在皮摩尔浓度范围内对凝血酶进行定量,线性响应高达 2.5 pM,检测限为 0.05 pM。该方法用于测定人血浆中的凝血酶活性和凝血酶抑制剂阿加曲班。图形摘要 用于测定皮摩尔浓度范围内凝血酶活性的荧光法的示意图,该方法基于纤维蛋白原与凝血酶活化后的相互作用,以及由 CdS 量子点 (CdS QD) 原位生长组成的酶促放大步骤。基于与凝血酶活化后纤维蛋白原之间的相互作用,以及由 CdS 量子点 (CdS QD) 原位生长组成的酶促放大步骤,用于测定皮摩尔浓度范围内凝血酶活性的荧光法的示意图。
更新日期:2019-08-29
中文翻译:

原位生成的 CdS 量子点用于凝血酶活性的荧光测定
提出了一种灵敏测定凝血酶活性的方法。它基于 (a) 用凝血酶激活后纤维蛋白原之间的相互作用,以及 (b) 由 CdS 量子点 (QD) 原位生长组成的酶促放大步骤。纤维蛋白原固定在微孔板的孔表面,然后与生物素化纤维蛋白原和凝血酶的混合物一起孵育。凝血酶激活固定的纤维蛋白原和游离的生物素化纤维蛋白原。这导致形成不溶性生物素化纤维蛋白,其仍结合在孔表面。然后,将样品与与生物素化纤维蛋白结合的亲和素标记的碱性磷酸酶 (ALP) 一起孵育。ALP 水解底物对硝基苯基磷酸酯 (pNPP),形成磷酸根离子,稳定由镉 (II) 和硫化物原位生长的 CdS QD。纤维蛋白的产生与凝血酶的活性有关。凝血酶浓度增加导致荧光增加,可在 300/510 nm 的激发/发射波长下测量。这种扩增步骤(QD 的酶触发生长)的引入允许在皮摩尔浓度范围内对凝血酶进行定量,线性响应高达 2.5 pM,检测限为 0.05 pM。该方法用于测定人血浆中的凝血酶活性和凝血酶抑制剂阿加曲班。图形摘要 用于测定皮摩尔浓度范围内凝血酶活性的荧光法的示意图,该方法基于纤维蛋白原与凝血酶活化后的相互作用,以及由 CdS 量子点 (CdS QD) 原位生长组成的酶促放大步骤。基于与凝血酶活化后纤维蛋白原之间的相互作用,以及由 CdS 量子点 (CdS QD) 原位生长组成的酶促放大步骤,用于测定皮摩尔浓度范围内凝血酶活性的荧光法的示意图。






























 京公网安备 11010802027423号
京公网安备 11010802027423号