当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of Fluorogenic Substrates of α-l-Fucosidase Useful for Inhibitor Screening and Gene-expression Profiling.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-08-29 , DOI: 10.1021/acsmedchemlett.9b00259 Kazuki Miura 1 , Takumi Tsukagoshi 1 , Takako Hirano 1 , Toshiyuki Nishio 1 , Wataru Hakamata 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2019-08-29 , DOI: 10.1021/acsmedchemlett.9b00259 Kazuki Miura 1 , Takumi Tsukagoshi 1 , Takako Hirano 1 , Toshiyuki Nishio 1 , Wataru Hakamata 1
Affiliation
|
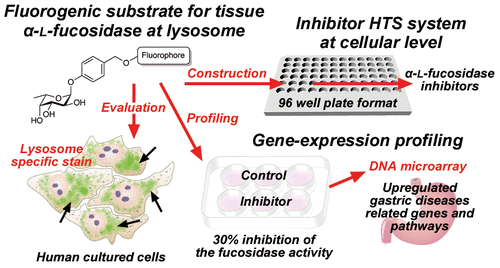
Inhibitors of human α-l-fucosidases, tissue α-l-fucosidase (tFuc), and plasma α-l-fucosidase reportedly play roles in multiple diseases, suggesting their therapeutic potential for gastric disease associated with Helicobacter pylori and fucosidosis. Terminal fucose linkages on glycoproteins and glycolipids are a natural substrate for both enzymes; however, there are currently no fluorogenic substrates allowing their cellular evaluation. Here, we described the development of novel three-color fluorogenic substrates for lysosome-localized tFuc that exhibited excellent specificity and sensitivity in three human cell lines. Additionally, we developed a cell-based high-throughput inhibitor screening system in a 96-well format and a cell-based inhibitory activity evaluation system in a 6-well format for tFuc inhibitors using this substrate, which allowed accurate quantification of the inhibition rate. Moreover, analysis of significant changes in gene expression resulting from 30% inhibition of tFuc in HeLa cells revealed potential roles in gastric disease.
中文翻译:

用于抑制剂筛选和基因表达谱分析的α-1-岩藻糖苷酶荧光底物的开发。
据报道,人α- 1-岩藻糖苷酶,组织α- 1-岩藻糖苷酶(tFuc)和血浆α- 1-岩藻糖苷酶的抑制剂在多种疾病中均起作用,表明它们具有治疗幽门螺杆菌相关胃病的潜力和岩藻病。糖蛋白和糖脂上的末端岩藻糖键是两种酶的天然底物。但是,目前尚无可用于细胞评估的荧光底物。在这里,我们描述了针对溶酶体定位的tFuc的新型三色荧光底物的开发,该底物在三种人类细胞系中均表现出优异的特异性和敏感性。此外,我们使用该底物为tFuc抑制剂开发了96孔形式的基于细胞的高通量抑制剂筛选系统和6孔形式的基于细胞的抑制活性评估系统,从而可以准确定量抑制率。此外,对HeLa细胞中tFuc的30%抑制产生的基因表达显着变化的分析显示,其在胃病中可能发挥作用。
更新日期:2019-08-29
中文翻译:

用于抑制剂筛选和基因表达谱分析的α-1-岩藻糖苷酶荧光底物的开发。
据报道,人α- 1-岩藻糖苷酶,组织α- 1-岩藻糖苷酶(tFuc)和血浆α- 1-岩藻糖苷酶的抑制剂在多种疾病中均起作用,表明它们具有治疗幽门螺杆菌相关胃病的潜力和岩藻病。糖蛋白和糖脂上的末端岩藻糖键是两种酶的天然底物。但是,目前尚无可用于细胞评估的荧光底物。在这里,我们描述了针对溶酶体定位的tFuc的新型三色荧光底物的开发,该底物在三种人类细胞系中均表现出优异的特异性和敏感性。此外,我们使用该底物为tFuc抑制剂开发了96孔形式的基于细胞的高通量抑制剂筛选系统和6孔形式的基于细胞的抑制活性评估系统,从而可以准确定量抑制率。此外,对HeLa细胞中tFuc的30%抑制产生的基因表达显着变化的分析显示,其在胃病中可能发挥作用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号