当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Influence of the distal guanidine group on the rate and selectivity of O2 reduction by iron porphyrin.
Chemical Science ( IF 7.6 ) Pub Date : 2019-08-29 , DOI: 10.1039/c9sc02711d Arnab Ghatak 1 , Snehadri Bhakta 1 , Sarmistha Bhunia 1 , Abhishek Dey 1
Chemical Science ( IF 7.6 ) Pub Date : 2019-08-29 , DOI: 10.1039/c9sc02711d Arnab Ghatak 1 , Snehadri Bhakta 1 , Sarmistha Bhunia 1 , Abhishek Dey 1
Affiliation
|
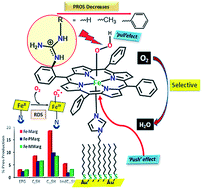
The O2 reduction reaction (ORR) catalysed by iron porphyrins with covalently attached pendant guanidine groups is reported. The results show a clear enhancement in the rate and selectivity for the 4e-/4H+ ORR. In situ resonance Raman investigations show that the rate determining step (rds) is O2 binding to ferrous porphyrins in contrast to the case of mononuclear iron porphyrins and heme/Cu analogues where the O-O bond cleavage of a heme peroxide is the rds. The selectivity is further enhanced when an axial imidazole ligand is introduced. Thus, the combination of the axial imidazole ligand and pendant guanidine ligand, analogous to the active site of peroxidases, is determined to be very effective in enabling a facile and selective 4e-/4H+ ORR.
中文翻译:

远端胍基团对铁卟啉还原 O2 的速率和选择性的影响。
报道了由具有共价连接的悬垂胍基团的铁卟啉催化的 O2 还原反应 (ORR)。结果显示 4e-/4H+ ORR 的速率和选择性明显增强。原位共振拉曼研究表明,速率决定步骤 (rds) 是 O2 与亚铁卟啉结合,与单核铁卟啉和血红素/铜类似物的情况相反,其中血红素过氧化物的 OO 键裂解是 rds。当引入轴向咪唑配体时,选择性进一步增强。因此,类似于过氧化物酶的活性位点,轴向咪唑配体和悬垂胍配体的组合被确定对于实现简便且选择性的 4e-/4H+ ORR 非常有效。
更新日期:2019-11-01
中文翻译:

远端胍基团对铁卟啉还原 O2 的速率和选择性的影响。
报道了由具有共价连接的悬垂胍基团的铁卟啉催化的 O2 还原反应 (ORR)。结果显示 4e-/4H+ ORR 的速率和选择性明显增强。原位共振拉曼研究表明,速率决定步骤 (rds) 是 O2 与亚铁卟啉结合,与单核铁卟啉和血红素/铜类似物的情况相反,其中血红素过氧化物的 OO 键裂解是 rds。当引入轴向咪唑配体时,选择性进一步增强。因此,类似于过氧化物酶的活性位点,轴向咪唑配体和悬垂胍配体的组合被确定对于实现简便且选择性的 4e-/4H+ ORR 非常有效。































 京公网安备 11010802027423号
京公网安备 11010802027423号