当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Studies on Phase Equilibria in the Ternary System LiCl–SrCl2–H2O and the Quaternary System KCl–LiCl–SrCl2–H2O at 308 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-08-27 , DOI: 10.1021/acs.jced.9b00421 Xiao-Ping Li 1 , Chun-Xia He 1 , Yun-Yun Gao 1 , Xiao-Feng He 1 , Shi-Hua Sang 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2019-08-27 , DOI: 10.1021/acs.jced.9b00421 Xiao-Ping Li 1 , Chun-Xia He 1 , Yun-Yun Gao 1 , Xiao-Feng He 1 , Shi-Hua Sang 1, 2
Affiliation
|
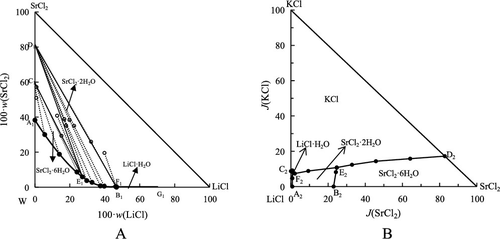
The ternary system LiCl–SrCl2–H2O and the quaternary system KCl–LiCl–SrCl2–H2O at 308 K were investigated using the isothermal dissolution equilibrium method. According to the obtained experimental results, the phase diagrams of the two systems listed above were plotted separately. The results show that both systems belong to hydrate I-type cosaturated systems at 308 K, and there are no double salts or solid solution formation. Lithium chloride has strong salting-out effect on strontium chloride and potassium chloride. There are two invariant points, three univariate curves, and three crystallization zones of SrCl2·6H2O, SrCl2·2H2O, and LiCl·H2O in the ternary system LiCl–SrCl2–H2O at 308 K. The equilibrium phase diagram of the quaternary system KCl–LiCl–SrCl2–H2O at 308 K consists of two invariant points, five univariant curves, and four crystallization zones, which correspond to SrCl2·6H2O, SrCl2·2H2O, LiCl·H2O, and KCl. These results can provide solubility data for the development and utilization of the underground brine in the Sichuan Basin of China.
更新日期:2019-08-28


















































 京公网安备 11010802027423号
京公网安备 11010802027423号