当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of a bound peptide phosphonate reveals the mechanism of nocardicin bifunctional thioesterase epimerase-hydrolase half-reactions.
Nature Communications ( IF 14.7 ) Pub Date : 2019-08-27 , DOI: 10.1038/s41467-019-11740-6 Ketan D Patel 1 , Felipe B d'Andrea 2, 3 , Nicole M Gaudelli 2, 4 , Andrew R Buller 2, 5 , Craig A Townsend 2 , Andrew M Gulick 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-08-27 , DOI: 10.1038/s41467-019-11740-6 Ketan D Patel 1 , Felipe B d'Andrea 2, 3 , Nicole M Gaudelli 2, 4 , Andrew R Buller 2, 5 , Craig A Townsend 2 , Andrew M Gulick 1
Affiliation
|
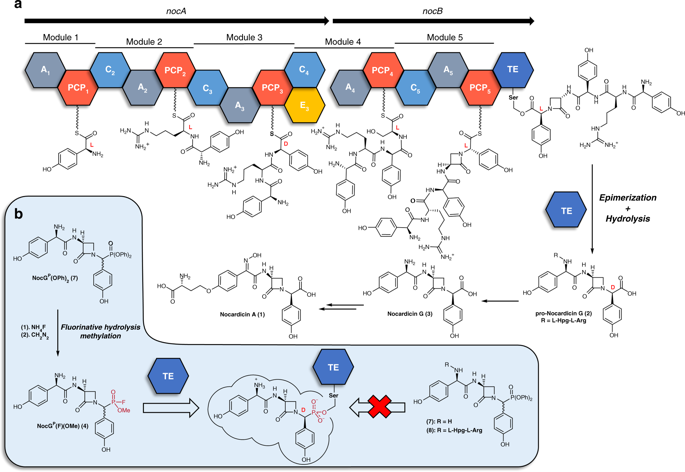
Nonribosomal peptide synthetases (NRPSs) underlie the biosynthesis of many natural products that have important medicinal utility. Protection of the NRPS peptide products from proteolysis is critical to these pathways and is often achieved by structural modification, principally the introduction of D-amino acid residues into the elongating peptide. These amino acids are generally formed in situ from their L-stereoisomers by epimerization domains or dual-function condensation/epimerization domains. In singular contrast, the thioesterase domain of nocardicin biosynthesis mediates both the effectively complete L- to D-epimerization of its C-terminal amino acid residue (≥100:1) and hydrolytic product release. We report herein high-resolution crystal structures of the nocardicin thioesterase domain in ligand-free form and reacted with a structurally precise fluorophosphonate substrate mimic that identify the complete peptide binding pocket to accommodate both stereoisomers. These structures combined with additional functional studies provide detailed mechanistic insight into this unique dual-function NRPS domain.
中文翻译:

结合的肽膦酸酯的结构揭示了诺卡汀双功能硫酯酶差向异构酶-水解酶半反应的机制。
非核糖体肽合成酶(NRPS)是许多具有重要医学用途的天然产物的生物合成基础。NRPS肽产物免受蛋白水解的保护对于这些途径至关重要,并且通常是通过结构修饰实现的,主要是将D-氨基酸残基引入延伸肽中。这些氨基酸通常由它们的L-立体异构体通过差向异构域或双功能缩合/表观异构域原位形成。与之形成鲜明对比的是,诺卡霉素生物合成的硫酯酶结构域介导了其C端氨基酸残基(≥100:1)的有效完全L-至D-表异构化和水解产物释放。我们在这里报告诺卡汀硫酯酶域的高分辨率晶体结构,无配体形式,并与结构精确的氟代磷酸酯底物模拟物反应,该模拟物识别出完整的肽结合口袋,以适应两种立体异构体。这些结构与其他功能研究相结合,为这种独特的双功能NRPS域提供了详细的机械原理。
更新日期:2019-08-27
中文翻译:

结合的肽膦酸酯的结构揭示了诺卡汀双功能硫酯酶差向异构酶-水解酶半反应的机制。
非核糖体肽合成酶(NRPS)是许多具有重要医学用途的天然产物的生物合成基础。NRPS肽产物免受蛋白水解的保护对于这些途径至关重要,并且通常是通过结构修饰实现的,主要是将D-氨基酸残基引入延伸肽中。这些氨基酸通常由它们的L-立体异构体通过差向异构域或双功能缩合/表观异构域原位形成。与之形成鲜明对比的是,诺卡霉素生物合成的硫酯酶结构域介导了其C端氨基酸残基(≥100:1)的有效完全L-至D-表异构化和水解产物释放。我们在这里报告诺卡汀硫酯酶域的高分辨率晶体结构,无配体形式,并与结构精确的氟代磷酸酯底物模拟物反应,该模拟物识别出完整的肽结合口袋,以适应两种立体异构体。这些结构与其他功能研究相结合,为这种独特的双功能NRPS域提供了详细的机械原理。































 京公网安备 11010802027423号
京公网安备 11010802027423号