Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacologically reversible, loss of function mutations in the TM2 and TM4 inner pore helices of TREK-1 K2P channels.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-08-27 , DOI: 10.1038/s41598-019-48855-1 Ehab Al-Moubarak 1 , Emma L Veale 1 , Alistair Mathie 1
Scientific Reports ( IF 3.8 ) Pub Date : 2019-08-27 , DOI: 10.1038/s41598-019-48855-1 Ehab Al-Moubarak 1 , Emma L Veale 1 , Alistair Mathie 1
Affiliation
|
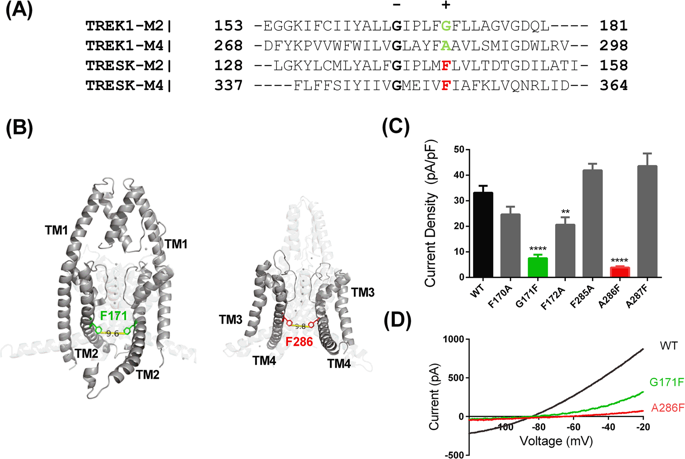
A better understanding of the gating of TREK two pore domain potassium (K2P) channels and their activation by compounds such as the negatively charged activator, flufenamic acid (FFA) is critical in the search for more potent and selective activators of these channels. Currents through wild-type and mutated human K2P channels expressed in tsA201 cells were measured using whole-cell patch-clamp recordings in the presence and absence of FFA. Mutation of the TM2.6 residue of TREK-1 to a phenylalanine (G171F) and a similar mutation of TM4.6 (A286F) substantially reduced current through TREK-1 channels. In complementary experiments, replacing the natural F residues at the equivalent position in TRESK channels, significantly enhanced current. Known, gain of function mutations of TREK-1 (G137I, Y284A) recovered current through these mutated channels. This reduction in current could be also be reversed pharmacologically, by FFA. However, an appropriate length MTS (MethaneThioSulfonate) cross-linking reagent (MTS14) restricted the activation of TREK-1_A286C channels by repeated application of FFA. This suggests that the cross-linker stabilises the channel in a conformation which blunts FFA activation. Pharmacologically reversible mutations of TREK channels will help to clarify the importance of these channels in pathophysiological conditions such as pain and depression.
中文翻译:

药理学上可逆的,TREK-1 K2P通道的TM2和TM4内部孔螺旋中的功能突变丢失。
更好地了解TREK两个孔结构域钾(K2P)通道的门控以及它们被诸如带负电荷的活化剂,氟芬那酸(FFA)之类的化合物活化的过程,对于寻找这些通道的更强效和选择性活化剂至关重要。在存在和不存在FFA的情况下,使用全细胞膜片钳记录来测量通过tsA201细胞表达的野生型和突变的人K2P通道的电流。TREK-1的TM2.6残基突变为苯丙氨酸(G171F)和TM4.6的类似突变(A286F)大大降低了通过TREK-1通道的电流。在补充实验中,替换TRESK通道中等效位置的天然F残基可显着增强电流。已知,TREK-1(G137I,Y284A)功能突变的获得通过这些突变的通道恢复了电流。FFA还可以通过药理学逆转电流的这种降低。但是,适当长度的MTS(甲烷硫代磺酸盐)交联剂(MTS14)通过重复应用FFA限制了TREK-1_A286C通道的激活。这表明交联剂以钝化FFA活化的构象稳定了通道。TREK通道的药理学可逆突变将有助于阐明这些通道在病理生理状况(如疼痛和抑郁)中的重要性。这表明交联剂以钝化FFA活化的构象稳定了通道。TREK通道的药理学可逆突变将有助于阐明这些通道在病理生理状况(如疼痛和抑郁)中的重要性。这表明交联剂以钝化FFA活化的构象稳定通道。TREK通道的药理学可逆突变将有助于阐明这些通道在病理生理状况(如疼痛和抑郁)中的重要性。
更新日期:2019-08-27
中文翻译:

药理学上可逆的,TREK-1 K2P通道的TM2和TM4内部孔螺旋中的功能突变丢失。
更好地了解TREK两个孔结构域钾(K2P)通道的门控以及它们被诸如带负电荷的活化剂,氟芬那酸(FFA)之类的化合物活化的过程,对于寻找这些通道的更强效和选择性活化剂至关重要。在存在和不存在FFA的情况下,使用全细胞膜片钳记录来测量通过tsA201细胞表达的野生型和突变的人K2P通道的电流。TREK-1的TM2.6残基突变为苯丙氨酸(G171F)和TM4.6的类似突变(A286F)大大降低了通过TREK-1通道的电流。在补充实验中,替换TRESK通道中等效位置的天然F残基可显着增强电流。已知,TREK-1(G137I,Y284A)功能突变的获得通过这些突变的通道恢复了电流。FFA还可以通过药理学逆转电流的这种降低。但是,适当长度的MTS(甲烷硫代磺酸盐)交联剂(MTS14)通过重复应用FFA限制了TREK-1_A286C通道的激活。这表明交联剂以钝化FFA活化的构象稳定了通道。TREK通道的药理学可逆突变将有助于阐明这些通道在病理生理状况(如疼痛和抑郁)中的重要性。这表明交联剂以钝化FFA活化的构象稳定了通道。TREK通道的药理学可逆突变将有助于阐明这些通道在病理生理状况(如疼痛和抑郁)中的重要性。这表明交联剂以钝化FFA活化的构象稳定通道。TREK通道的药理学可逆突变将有助于阐明这些通道在病理生理状况(如疼痛和抑郁)中的重要性。































 京公网安备 11010802027423号
京公网安备 11010802027423号