Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
AntimiR-155 Cyclic Peptide–PNA Conjugate: Synthesis, Cellular Uptake, and Biological Activity
ACS Omega ( IF 3.7 ) Pub Date : 2019-08-12 00:00:00 , DOI: 10.1021/acsomega.9b01697 Terese Soudah , Saleh Khawaled , Rami I. Aqeilan , Eylon Yavin
ACS Omega ( IF 3.7 ) Pub Date : 2019-08-12 00:00:00 , DOI: 10.1021/acsomega.9b01697 Terese Soudah , Saleh Khawaled , Rami I. Aqeilan , Eylon Yavin
|
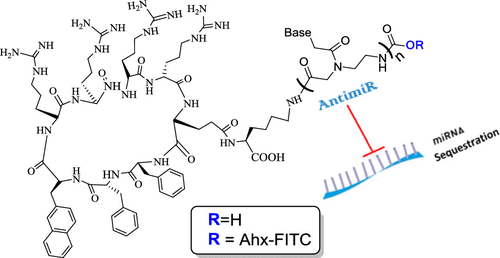
Efficient delivery of nucleic acids into cells still remains a great challenge. Peptide nucleic acids (PNAs) are DNA analogues with a neutral backbone and are synthesized by solid phase peptide chemistry. This allows a straightforward synthetic route to introduce a linear short peptide (a.k.a. cell-penetrating peptide) to the PNA molecule as a means of facilitating cellular uptake of PNAs. Herein, we have devised a synthetic route in which a cyclic peptide is prepared on a solid support and is extended with the PNA molecule, where all syntheses are accomplished on the solid phase. This allows the conjugation of the cyclic peptide to the PNA molecule with the need of only one purification step after the cyclic peptide–PNA conjugate (C9–PNA) is cleaved from the solid support. The PNA sequence chosen is an antimiR-155 molecule that is complementary to mature miR-155, a well-established oncogenic miRNA. By labeling C9–PNA with fluorescein isothiocyanate, we observe efficient cellular uptake into glioblastoma cells (U87MG) at a low concentration (0.5 μM), as corroborated by fluorescence-activated cell sorting (FACS) analysis and confocal microscopy. FACS analysis also suggests an uptake mechanism that is energy-dependent. Finally, the antimiR activity of C9–PNA was shown by analyzing miR155 levels by quantitative reverse transcription polymerase chain reaction and by observing a reduction in cell viability and proliferation in U87MG cells, as corroborated by XTT and colony formation assays. Given the added biological stability of cyclic versus linear peptides, this synthetic approach may be a useful and straightforward approach to synthesize cyclic peptide–PNA conjugates.
中文翻译:

AntimiR-155环肽-PNA偶联物:合成,细胞摄取和生物活性
将核酸有效地递送到细胞中仍然是巨大的挑战。肽核酸(PNA)是具有中性骨架的DNA类似物,是通过固相肽化学合成的。这允许直接合成路线将线性短肽(又称细胞穿透肽)引入PNA分子,作为促进细胞吸收PNA的手段。本文中,我们设计了一种合成路线,其中在固相支持物上制备环肽并与PNA分子一起延伸,其中所有合成均在固相上完成。这样就可以将环肽与PNA分子偶联,而在环肽-PNA偶联物之后只需一个纯化步骤(C 9–PNA)从固体支持物上裂解下来。选择的PNA序列是与成熟的miR-155(成熟的致癌miRNA)互补的antimiR-155分子。通过用异硫氰酸荧光素标记C 9 –PNA,我们观察到了低浓度(0.5μM)的胶质母细胞瘤细胞(U87MG)的有效细胞摄取,这被荧光激活细胞分选(FACS)分析和共聚焦显微镜证实。FACS分析还提出了一种依赖能量的摄取机制。最后,C 9的抗miR活性通过定量逆转录聚合酶链反应分析miR155水平并观察到U87MG细胞中细胞活力和增殖的降低,可以显示–PNA,这已通过XTT和集落形成分析得到了证实。考虑到环状肽与线性肽具有更高的生物稳定性,这种合成方法可能是合成环状肽-PNA缀合物的有用且直接的方法。
更新日期:2019-08-12
中文翻译:

AntimiR-155环肽-PNA偶联物:合成,细胞摄取和生物活性
将核酸有效地递送到细胞中仍然是巨大的挑战。肽核酸(PNA)是具有中性骨架的DNA类似物,是通过固相肽化学合成的。这允许直接合成路线将线性短肽(又称细胞穿透肽)引入PNA分子,作为促进细胞吸收PNA的手段。本文中,我们设计了一种合成路线,其中在固相支持物上制备环肽并与PNA分子一起延伸,其中所有合成均在固相上完成。这样就可以将环肽与PNA分子偶联,而在环肽-PNA偶联物之后只需一个纯化步骤(C 9–PNA)从固体支持物上裂解下来。选择的PNA序列是与成熟的miR-155(成熟的致癌miRNA)互补的antimiR-155分子。通过用异硫氰酸荧光素标记C 9 –PNA,我们观察到了低浓度(0.5μM)的胶质母细胞瘤细胞(U87MG)的有效细胞摄取,这被荧光激活细胞分选(FACS)分析和共聚焦显微镜证实。FACS分析还提出了一种依赖能量的摄取机制。最后,C 9的抗miR活性通过定量逆转录聚合酶链反应分析miR155水平并观察到U87MG细胞中细胞活力和增殖的降低,可以显示–PNA,这已通过XTT和集落形成分析得到了证实。考虑到环状肽与线性肽具有更高的生物稳定性,这种合成方法可能是合成环状肽-PNA缀合物的有用且直接的方法。





























 京公网安备 11010802027423号
京公网安备 11010802027423号