当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile synthesis of bromo- and mixed bromo/chloro dibenzo-p-dioxins and [14C]-labeled 1,3,7,8-tetrabromodibenzo-p-dioxin.
Chemosphere ( IF 8.1 ) Pub Date : 2019-08-27 , DOI: 10.1016/j.chemosphere.2019.124626 Anuradha Singh 1 , Heldur Hakk 1 , Sara Lupton 1
Chemosphere ( IF 8.1 ) Pub Date : 2019-08-27 , DOI: 10.1016/j.chemosphere.2019.124626 Anuradha Singh 1 , Heldur Hakk 1 , Sara Lupton 1
Affiliation
|
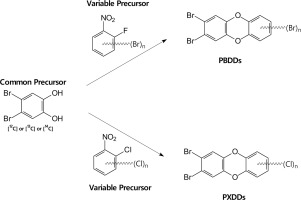
Polybrominated dibenzo-p-dioxins (PBDDs) and mixed bromo/chloro dibenzo-p-dioxins (PXDDs) are persistent organic pollutants that can possess the same toxicity as their fully chlorinated analogs (PCDDs) and have been identified in the same matrices. Herein a general synthetic methodology is described to produce multiple congeners of PBDDs and PXDDs with varying degrees of halogenation and substitution patterns for use as analytical and/or internal standards, and for absorption, disposition, metabolism, and excretion (ADME) studies. The syntheses of PBDDs and PXDDs were accomplished by condensing a common precursor, 4,5-dibromo catechol, with variable precursors, i.e., polychlorinated 1-chloro-2-nitrobenzenes or polybrominated 1-fluoro-2-nitrobenzenes, to introduce a desired number of halogens and specific substitution patterns. Initial attempts to synthesize PBDDs and PXDDs were performed in potassium carbonate with DMSO at 145-150 °C. PXDDs syntheses resulted in formation of the desired products at >90% purity but attempts at higher brominated PBDDs syntheses resulted in dehalogenated by-products. To preclude by-product formation, additional syntheses for some PBDDs were conducted by refluxing the precursors in acetonitrile, which resulted in pure products at higher yield. Six PXDDs ranging from four to six halogens were synthesized (20-84% yield), of which three contained the halogen substitution pattern of 2,3,7,8. Five PBDDs ranging from four to six bromines were produced in 23-83% yield, three of which were toxic. Using the initial DMSO method, [14C]-1,3,7,8-tetrabromodibenzo-p-dioxin (0.26 μCi/μmol; 11% overall yield) was synthesized from commercially available [14C]-phenol to allow an ADME study to be conducted.
中文翻译:

轻松合成溴和混合的溴/氯二苯并-对二恶英和[14C]标记的1,3,7,8-四溴二苯并-对二恶英。
多溴二苯并对二恶英(PBDDs)和溴/氯二苯并对二恶英混合(PXDDs)是持久性有机污染物,其毒性与其完全氯化的类似物(PCDDs)相同,并且已在同一基质中鉴定出来。在此描述了一种通用的合成方法,该方法可生产具有不同程度卤化和取代模式的PBDDs和PXDDs同系物,用作分析和/或内标,以及用于吸收,处置,代谢和排泄(ADME)研究。PBDD和PXDD的合成是通过将常见的前体4,5-二溴邻苯二酚与可变的前体(即多氯代1-氯-2-硝基苯或多溴代1-氟-2-硝基苯)缩合以引入所需数量而完成的。卤素和特定的取代模式。最初尝试在145-150°C的碳酸钾中用DMSO合成PBDDs和PXDDs。PXDDs的合成导致形成纯度> 90%的所需产物,但尝试更高溴化的PBDDs的合成会导致脱卤的副产物。为了防止副产物的形成,通过将前体在乙腈中回流,进行了一些多溴二苯并二恶英的额外合成,从而以较高的收率得到了纯净的产物。合成了六种PXDD,范围从4到6个卤素不等(收率20-84%),其中三个包含2,3,7,8的卤素取代图案。以23-83%的收率生产了5种多溴二苯并二恶英,溴含量从4到6种不等,其中三种有毒。使用最初的DMSO方法,[14C] -1,3,7,8-四溴二苯并-对二恶英(0.26μCi/μmol;
更新日期:2019-08-27
中文翻译:

轻松合成溴和混合的溴/氯二苯并-对二恶英和[14C]标记的1,3,7,8-四溴二苯并-对二恶英。
多溴二苯并对二恶英(PBDDs)和溴/氯二苯并对二恶英混合(PXDDs)是持久性有机污染物,其毒性与其完全氯化的类似物(PCDDs)相同,并且已在同一基质中鉴定出来。在此描述了一种通用的合成方法,该方法可生产具有不同程度卤化和取代模式的PBDDs和PXDDs同系物,用作分析和/或内标,以及用于吸收,处置,代谢和排泄(ADME)研究。PBDD和PXDD的合成是通过将常见的前体4,5-二溴邻苯二酚与可变的前体(即多氯代1-氯-2-硝基苯或多溴代1-氟-2-硝基苯)缩合以引入所需数量而完成的。卤素和特定的取代模式。最初尝试在145-150°C的碳酸钾中用DMSO合成PBDDs和PXDDs。PXDDs的合成导致形成纯度> 90%的所需产物,但尝试更高溴化的PBDDs的合成会导致脱卤的副产物。为了防止副产物的形成,通过将前体在乙腈中回流,进行了一些多溴二苯并二恶英的额外合成,从而以较高的收率得到了纯净的产物。合成了六种PXDD,范围从4到6个卤素不等(收率20-84%),其中三个包含2,3,7,8的卤素取代图案。以23-83%的收率生产了5种多溴二苯并二恶英,溴含量从4到6种不等,其中三种有毒。使用最初的DMSO方法,[14C] -1,3,7,8-四溴二苯并-对二恶英(0.26μCi/μmol;































 京公网安备 11010802027423号
京公网安备 11010802027423号