当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1‐Aryltriazenes in the Suzuki, Heck, and Sonogashira Reactions in Imidazolium‐ILs, with [BMIM(SO3H)][OTf] or Sc(OTf)3 as Promoter, and Pd(OAc)2 or NiCl2·glyme as Catalyst
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-09-11 , DOI: 10.1002/ejoc.201901070 Suraj M. Sutar 1 , Hemantkumar M. Savanur 1 , Shruti S. Malunavar 1 , Pavankumar Prabhala 1 , Rajesh G. Kalkhambkar 1 , Kenneth K. Laali 2
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-09-11 , DOI: 10.1002/ejoc.201901070 Suraj M. Sutar 1 , Hemantkumar M. Savanur 1 , Shruti S. Malunavar 1 , Pavankumar Prabhala 1 , Rajesh G. Kalkhambkar 1 , Kenneth K. Laali 2
Affiliation
|
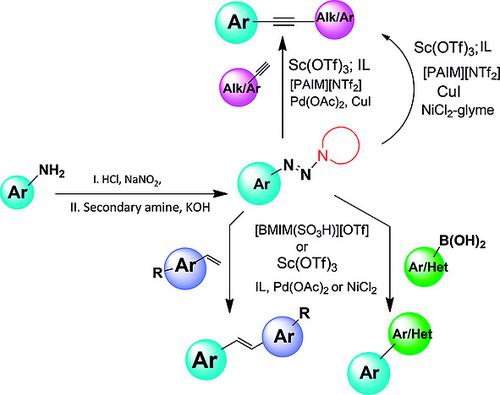
Acting as surrogates for aryldiazonium, 1‐aryltriazenes are readily unmasked with [BMIM(SO3H)][OTf] or Sc(OTf)3 and enter into cross‐coupling in widely available imidazolium‐ILs as solvent. The scope of this chemistry in the Suzuki, Heck, and Sonogashira reactions is explored. The reactions give good isolated yields, employing either Pd or Ni as catalyst and thus widening the scope of the protocol.

中文翻译:

咪唑鎓ILs中Suzuki,Heck和Sonogashira反应中的1-Aryltriazenes,以[BMIM(SO3H)] [OTf]或Sc(OTf)3为促进剂,并以Pd(OAc)2或NiCl2·甘醇二甲醚为催化剂
1-芳基三氮烯作为芳基重氮的替代物,很容易被[BMIM(SO 3 H)] [OTf]或Sc(OTf)3掩盖,并在广泛可用的咪唑-ILs中作为溶剂进行交叉偶联。探索了该化学在铃木,Heck和Sonogashira反应中的范围。使用Pd或Ni作为催化剂,反应可得到良好的分离收率,从而扩大了方案的范围。

更新日期:2019-09-11

中文翻译:

咪唑鎓ILs中Suzuki,Heck和Sonogashira反应中的1-Aryltriazenes,以[BMIM(SO3H)] [OTf]或Sc(OTf)3为促进剂,并以Pd(OAc)2或NiCl2·甘醇二甲醚为催化剂
1-芳基三氮烯作为芳基重氮的替代物,很容易被[BMIM(SO 3 H)] [OTf]或Sc(OTf)3掩盖,并在广泛可用的咪唑-ILs中作为溶剂进行交叉偶联。探索了该化学在铃木,Heck和Sonogashira反应中的范围。使用Pd或Ni作为催化剂,反应可得到良好的分离收率,从而扩大了方案的范围。






















































 京公网安备 11010802027423号
京公网安备 11010802027423号