当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Appended S-Block Metal Ion Crown Ethers on Redox Properties and Catalytic Activity of Mn–Nitride Schiff Base Complexes: Methane Activation
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-08-26 00:00:00 , DOI: 10.1021/acs.inorgchem.9b01696
Ahmad Najafian 1 , Thomas R. Cundari 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-08-26 00:00:00 , DOI: 10.1021/acs.inorgchem.9b01696
Ahmad Najafian 1 , Thomas R. Cundari 1
Affiliation
|
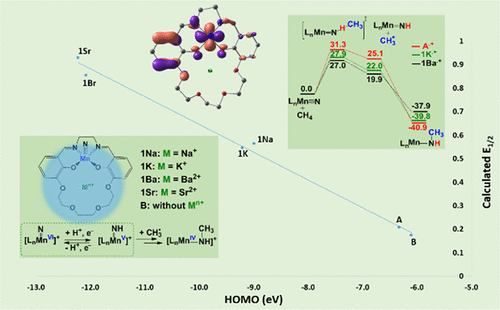
Using density functional theory (DFT), the effects of appended s-block metal ion crown ethers upon the redox properties of the following nitridomanganese(V) salen complexes were investigated: [(salen)MnV(N)(Mn+-crown ether)]n+ (salen = N,N′-bis(salicydene)ethylenediamine; M = Na+, K+, Ba2+, and Sr2+ for 1Na, 1K, 1Ba, and 1Sr, respectively; A = complex without Mn+-crown ether and B = without Mn+). NBO analysis of the MnN bond orders, optimized bond lengths, and stretching frequencies changes upon oxidation for all species show that for A, B, and 1Na MnN has more nitridyl character while a nitride form is more significant for 1K, 1Ba, and 1Sr. The results reveal that ΔGrxn(e–) and thus E1/2 are quite sensitive to the point charge (q) of the s-block metal ions (1 for K+/Na+ and 2 for Ba2+/Sr2+). Computations suggest that the degree of delocalization of the HOMO electrons on the supporting ligand is modified by the chelated s-block metal ion. Methane activation by A•+, 1K•+, and 1Ba•+ complexes proceeds via a hydrogen atom transfer (HAT) pathway with reasonable barriers for all complexes with ∼4 kcal/mol difference in energy. The molecular electrostatic potential (MEP) maps indicate a shift in redox potential imposed by the nonredox active cations by altering the electrostatic potential of the complexes. Computations show that the complexes with higher point charge of the incorporated metal ions result in higher N–H bond BDFEs. Changes in predicted properties as a function of continuum solvent dielectric constant suggest that the primary effect of the appended s-block ion is via “through space” interactions.
中文翻译:

附加的S嵌段金属离子冠醚对Mn-氮化物Schiff碱配合物的氧化还原性质和催化活性的影响:甲烷活化
使用密度泛函理论(DFT),研究了附加的s-嵌段金属离子冠醚对以下硝化锰(V)salen配合物的氧化还原性能的影响:[(salen)Mn V(N)(M n +-冠醚)] n +(salen = N,N'-双(水杨烯基)乙二胺; M = Na +,K +,Ba 2+和Sr 2+分别对应于1Na,1K,1Ba和1Sr ; A =络合物没有M n +-冠醚和B=不包含M n +)。对所有物种的MnN键序,优化的键长和拉伸频率进行的NBO分析表明,对于A,B和1Na而言, MnN具有更多的亚硝酰基特征,而1K,1Ba和1Sr的氮化物形式更为显着。结果表明,ΔG rxn(e –)和E 1/2对s嵌段金属离子的点电荷(q)十分敏感(K + / Na +为1,Ba 2+ / Sr为2 2+)。计算表明,HOMO电子在支持配体上的离域程度被螯合的s嵌段金属离子修饰。A •+,1K •+和1Ba •+活化甲烷络合物通过氢原子转移(HAT)途径进行,对所有能量差异约为4 kcal / mol的络合物都具有合理的势垒。分子静电势(MEP)图表明通过改变配合物的静电势,由非氧化还原活性阳离子施加的氧化还原电势发生了变化。计算表明,所掺入的金属离子具有较高的点电荷的配合物会导致较高的N–H键BDFE。预测特性随连续溶剂介电常数的变化表明,附加的s嵌段离子的主要作用是通过“穿越空间”相互作用。
更新日期:2019-08-26
中文翻译:

附加的S嵌段金属离子冠醚对Mn-氮化物Schiff碱配合物的氧化还原性质和催化活性的影响:甲烷活化
使用密度泛函理论(DFT),研究了附加的s-嵌段金属离子冠醚对以下硝化锰(V)salen配合物的氧化还原性能的影响:[(salen)Mn V(N)(M n +-冠醚)] n +(salen = N,N'-双(水杨烯基)乙二胺; M = Na +,K +,Ba 2+和Sr 2+分别对应于1Na,1K,1Ba和1Sr ; A =络合物没有M n +-冠醚和B=不包含M n +)。对所有物种的MnN键序,优化的键长和拉伸频率进行的NBO分析表明,对于A,B和1Na而言, MnN具有更多的亚硝酰基特征,而1K,1Ba和1Sr的氮化物形式更为显着。结果表明,ΔG rxn(e –)和E 1/2对s嵌段金属离子的点电荷(q)十分敏感(K + / Na +为1,Ba 2+ / Sr为2 2+)。计算表明,HOMO电子在支持配体上的离域程度被螯合的s嵌段金属离子修饰。A •+,1K •+和1Ba •+活化甲烷络合物通过氢原子转移(HAT)途径进行,对所有能量差异约为4 kcal / mol的络合物都具有合理的势垒。分子静电势(MEP)图表明通过改变配合物的静电势,由非氧化还原活性阳离子施加的氧化还原电势发生了变化。计算表明,所掺入的金属离子具有较高的点电荷的配合物会导致较高的N–H键BDFE。预测特性随连续溶剂介电常数的变化表明,附加的s嵌段离子的主要作用是通过“穿越空间”相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号