Nature Chemistry ( IF 19.2 ) Pub Date : 2019-08-23 , DOI: 10.1038/s41557-019-0314-x Xiaolong Sun 1, 2 , Brette M Chapin 2 , Pedro Metola 2 , Byron Collins 2 , Binghe Wang 3 , Tony D James 4 , Eric V Anslyn 2
|
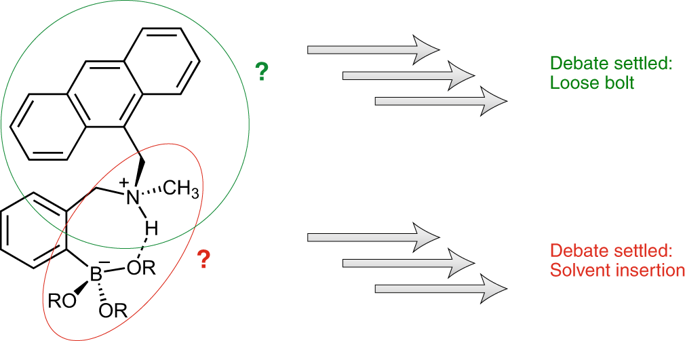
ortho-Aminomethylphenylboronic acids are used in receptors for carbohydrates and various other compounds containing vicinal diols. The presence of the o-aminomethyl group enhances the affinity towards diols at neutral pH, and the manner in which this group plays this role has been a topic of debate. Further, the aminomethyl group is believed to be involved in the turn-on of the emission properties of appended fluorophores upon diol binding. In this treatise, a uniform picture emerges for the role of this group: it primarily acts as an electron-withdrawing group that lowers the pKa of the neighbouring boronic acid thereby facilitating diol binding at neutral pH. The amine appears to play no role in the modulation of the fluorescence of appended fluorophores in the protic-solvent-inserted form of the boronic acid/boronate ester. Instead, fluorescence turn-on can be consistently tied to vibrational-coupled excited-state relaxation (a loose-bolt effect). Overall, this Review unifies and discusses the existing data as of 2019 whilst also highlighting why o-aminomethyl groups are so widely used, and the role they play in carbohydrate sensing using phenylboronic acids.
中文翻译:

邻氨基甲基苯基硼酸中硼酸酯形成和荧光开启的机制。
邻氨基甲基苯基硼酸用于碳水化合物和各种其他含有邻位二醇的化合物的受体。邻氨基甲基的存在增强了中性 pH 条件下对二醇的亲和力,该基团发挥这一作用的方式一直是争论的话题。此外,氨甲基被认为参与二醇结合后附加荧光团的发射特性的开启。在这篇论文中,该基团的作用得到了统一的描述:它主要充当吸电子基团,降低邻近硼酸的pKa ,从而促进二醇在中性 pH 下结合。在硼酸/硼酸酯的质子溶剂插入形式中,胺似乎对调节附加荧光团的荧光没有作用。相反,荧光开启可以始终与振动耦合激发态弛豫(松螺栓效应)联系在一起。总体而言,本综述统一并讨论了截至 2019 年的现有数据,同时还强调了邻氨甲基为何如此广泛使用,以及它们在使用苯基硼酸的碳水化合物传感中所发挥的作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号