当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Atherosclerosis is exacerbated by chitinase-3-like-1 in amyloid precursor protein transgenic mice
Theranostics ( IF 12.4 ) Pub Date : 2018-01-01 , DOI: 10.7150/thno.20183 Yu Yeon Jung , Ki Cheon Kim , Mi Hee Park , Youngsik Seo , Heonyong Park , Min Hee Park , Jun Chang , Dae Youn Hwang , Sang Bae Han , Sanghyeon Kim , Dong Ju Son , Jin Tae Hong
Theranostics ( IF 12.4 ) Pub Date : 2018-01-01 , DOI: 10.7150/thno.20183 Yu Yeon Jung , Ki Cheon Kim , Mi Hee Park , Youngsik Seo , Heonyong Park , Min Hee Park , Jun Chang , Dae Youn Hwang , Sang Bae Han , Sanghyeon Kim , Dong Ju Son , Jin Tae Hong
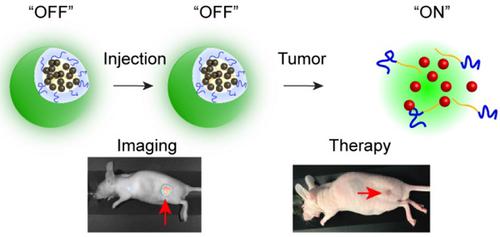
|
Although the important role of amyloid precursor protein (APP) in vascular diseases associated with Alzheimer's disease (AD) has been demonstrated, the underlying molecular mechanisms and physiological consequences are unclear. We aimed to evaluate vascular inflammation and atherosclerosis in Swedish mutant of human APP transgenic (APPsw-Tg) and ApoE-/-/APPsw-Tg mice. We also aimed to explore the mechanisms underlying any changes observed in these mice compared with non-Tg controls. Methods: The transgenic and non-Tg mouse strains were subjected to partial ligation of the left carotid artery to induce atherosclerotic changes, which were measured using histological approaches, immunohistochemistry, quantitative polymerase chain reaction, and gene expression microarrays. Results: Our results showed increased vascular inflammation, arterial wall thickness, and atherosclerosis in APPsw-Tg and ApoE-/-/APPsw-Tg mice. We further found that the expression of chitinase-3-like-1 (Chi3l1) is increased in the APPsw-Tg mouse artery and Chi3l1 mediates endothelial cell (EC) inflammation and vascular smooth muscle cell (VSMC) activation, which in turn exacerbates atherosclerosis. In addition, using two publicly available microarray datasets from the dorsolateral prefrontal cortex of people with AD and unaffected controls as well as inflamed human umbilical vein endothelial cells, we found that Chi3l1 and associated inflammatory gene were significantly associated with AD, evaluated by co-expression network analysis and functional annotation. Knockdown of Chi3l1 in the arterial endothelium in vivo suppressed the development of atherosclerosis. We also show that microRNA 342-3p (miR-342-3p) inhibits EC inflammation and VSMC activation through directly targeting Chi3l1, and that APPsw increased Chi3l1 expression by reducing miR-342-3p expression in the arterial endothelium, promoting atherosclerosis. Conclusion: Our findings suggest that targeting Chi3l1 might provide new diagnostic and therapeutic strategies for vascular diseases in patients with AD.
中文翻译:

淀粉样前体蛋白转基因小鼠中的几丁质酶-3-like-1加剧了动脉粥样硬化
尽管已经证明了淀粉样蛋白前体蛋白(APP)在与阿尔茨海默氏病(AD)相关的血管疾病中的重要作用,但潜在的分子机制和生理后果尚不清楚。我们旨在评估人类APP转基因(APPsw-Tg)和ApoE -/- / APPsw-Tg小鼠的瑞典突变体中的血管炎症和动脉粥样硬化。我们还旨在探索与非Tg对照相比在这些小鼠中观察到的任何变化的潜在机制。方法:将转基因小鼠和非Tg小鼠品系的左颈动脉部分结扎,以诱发动脉粥样硬化变化,并使用组织学方法,免疫组织化学,定量聚合酶链反应和基因表达微阵列进行测量。结果:我们的结果显示APPsw-Tg和ApoE中的血管炎症,动脉壁厚度增加和动脉粥样硬化-/-/ APPsw-Tg小鼠。我们进一步发现,几丁质酶-3-like-1(Chi3l1)的表达在APPsw-Tg小鼠动脉中增加,并且Chi3l1介导内皮细胞(EC)炎症和血管平滑肌细胞(VSMC)激活,进而加剧了动脉粥样硬化。此外,使用来自AD患者和未受影响对照者的背外侧前额叶皮层以及发炎的人脐静脉内皮细胞的两个可公开获得的微阵列数据集,我们发现Chi3l1和相关的炎性基因与AD显着相关,通过共表达进行评估网络分析和功能注释。Chi3l1在体内的内皮细胞的敲低抑制动脉粥样硬化的发展。我们还显示,microRNA 342-3p(miR-342-3p)通过直接靶向Chi3l1抑制EC炎症和VSMC激活,并且APPsw通过减少动脉内皮中的miR-342-3p表达来增加Chi3l1表达,从而促进动脉粥样硬化。结论:我们的发现表明,靶向Chi3l1可能为AD患者的血管疾病提供新的诊断和治疗策略。
更新日期:2018-03-01
中文翻译:

淀粉样前体蛋白转基因小鼠中的几丁质酶-3-like-1加剧了动脉粥样硬化
尽管已经证明了淀粉样蛋白前体蛋白(APP)在与阿尔茨海默氏病(AD)相关的血管疾病中的重要作用,但潜在的分子机制和生理后果尚不清楚。我们旨在评估人类APP转基因(APPsw-Tg)和ApoE -/- / APPsw-Tg小鼠的瑞典突变体中的血管炎症和动脉粥样硬化。我们还旨在探索与非Tg对照相比在这些小鼠中观察到的任何变化的潜在机制。方法:将转基因小鼠和非Tg小鼠品系的左颈动脉部分结扎,以诱发动脉粥样硬化变化,并使用组织学方法,免疫组织化学,定量聚合酶链反应和基因表达微阵列进行测量。结果:我们的结果显示APPsw-Tg和ApoE中的血管炎症,动脉壁厚度增加和动脉粥样硬化-/-/ APPsw-Tg小鼠。我们进一步发现,几丁质酶-3-like-1(Chi3l1)的表达在APPsw-Tg小鼠动脉中增加,并且Chi3l1介导内皮细胞(EC)炎症和血管平滑肌细胞(VSMC)激活,进而加剧了动脉粥样硬化。此外,使用来自AD患者和未受影响对照者的背外侧前额叶皮层以及发炎的人脐静脉内皮细胞的两个可公开获得的微阵列数据集,我们发现Chi3l1和相关的炎性基因与AD显着相关,通过共表达进行评估网络分析和功能注释。Chi3l1在体内的内皮细胞的敲低抑制动脉粥样硬化的发展。我们还显示,microRNA 342-3p(miR-342-3p)通过直接靶向Chi3l1抑制EC炎症和VSMC激活,并且APPsw通过减少动脉内皮中的miR-342-3p表达来增加Chi3l1表达,从而促进动脉粥样硬化。结论:我们的发现表明,靶向Chi3l1可能为AD患者的血管疾病提供新的诊断和治疗策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号