当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Spirocyclic Oxindole δ-Lactams via NHC-Catalyzed Formal [2+4] Annulation of Aliphatic Aldehydes with Oxindole-Derived α,β-Unsaturated Ketimines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-09-04 , DOI: 10.1021/acs.joc.9b01760 Chonglong He 1 , Zhanhuan Li 1 , Jianfeng Xu 1 , Hongjun Ren 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-09-04 , DOI: 10.1021/acs.joc.9b01760 Chonglong He 1 , Zhanhuan Li 1 , Jianfeng Xu 1 , Hongjun Ren 2
Affiliation
|
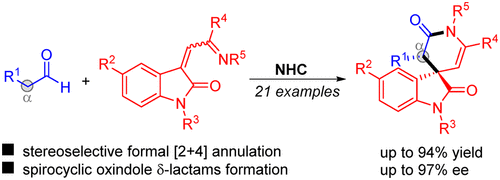
An N-heterocyclic carbene (NHC)-catalyzed formal [2+4] annulation reaction of aliphatic aldehydes with oxindole-derived α,β-unsaturated ketimines under oxidative conditions is reported, affording spirocyclic oxindole δ-lactams with good yields, moderate diastereoselectivies, and good to excellent enantioselectivies. This reaction can be readily carried out on a gram scale, and the products could be further transformed to other synthetically useful compounds.
中文翻译:

通过NHC催化的醛基衍生的α,β-不饱和酮亚胺形式的脂族醛的[2 + 4]环状不对称合成螺环氧吲哚δ-内酰胺
据报道,在氧化条件下,N-杂环卡宾(NHC)催化脂肪醛与甲醛衍生的α,β-不饱和酮亚胺的正式[2 + 4]环化反应,提供了具有良好收率,中等非对映选择性的螺环羟吲哚δ-内酰胺。并具有出色的对映选择性。该反应可以以克为单位容易地进行,并且产物可以进一步转化为其他合成上有用的化合物。
更新日期:2019-09-04
中文翻译:

通过NHC催化的醛基衍生的α,β-不饱和酮亚胺形式的脂族醛的[2 + 4]环状不对称合成螺环氧吲哚δ-内酰胺
据报道,在氧化条件下,N-杂环卡宾(NHC)催化脂肪醛与甲醛衍生的α,β-不饱和酮亚胺的正式[2 + 4]环化反应,提供了具有良好收率,中等非对映选择性的螺环羟吲哚δ-内酰胺。并具有出色的对映选择性。该反应可以以克为单位容易地进行,并且产物可以进一步转化为其他合成上有用的化合物。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号