Chem ( IF 19.1 ) Pub Date : 2019-08-22 , DOI: 10.1016/j.chempr.2019.07.023 Kai Liu , Nian Li , Yunyun Ning , Chengjian Zhu , Jin Xie
|
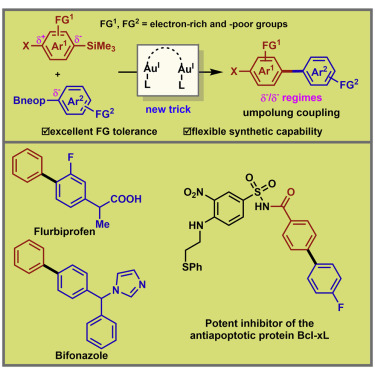
The biaryl coupling between a nucleophile (Arδ−: arylboronates or arylsilanes) and an electrophile (Arδ+: arylhalides) represents the state of the art in carbon–carbon bond formation. The intrinsic functional-group limitations in these reactions stem from the high catalytic reactivity of palladium and nickel catalysts toward halogen, boronate, and base-sensitive substituents. Here, we report a general dimeric gold-catalyzed oxidative cross-coupling of arylboronates and arylsilanes without an external base for the synthesis, with excellent functional-group tolerance of asymmetric biaryls. Both coupling partners are readily available, bench-stable, and non-toxic. A broad array of (pseudo)halogenated and borylated coupling partners can be successfully applied to this site-specific biaryl coupling with unprecedented versatility. Its synthetic value has been substantiated by concise preparation of several π-conjugated organic materials and pharmacophores.
中文翻译:

金催化有机金属的氧化联芳基交叉偶联
联芳基亲核试剂之间的偶联(AR δ-:arylboronates或芳基硅烷)和亲电(AR δ+:芳基卤化物)代表了碳-碳键形成的最新技术。这些反应中固有的官能团限制源于钯和镍催化剂对卤素,硼酸酯和碱敏感取代基的高催化反应性。在这里,我们报告了一般的二聚体金催化的芳基硼酸酯和芳基硅烷的氧化交叉偶联反应,而没有用于合成的外部碱,具有不对称联芳基的优异的官能团耐受性。两种偶合剂均易于获得,稳定且无毒。各种各样的(假)卤代和硼化偶合剂可以成功地应用于这种位点特定的联芳基偶合剂,具有前所未有的多功能性。简明地制备几种π共轭有机材料和药效基团已证实了其合成价值。































 京公网安备 11010802027423号
京公网安备 11010802027423号