当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Radical C-C Bond Cleavage/Addition Cascade of Benzyl Cycloketone Oxime Ethers Enabled by Photogenerated Cyclic Iminyl Radicals.
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-22 00:00:00 , DOI: 10.1021/acs.orglett.9b02535 Peng-Zi Wang 1 , Bin-Qing He 1 , Ying Cheng 1 , Jia-Rong Chen 1 , Wen-Jing Xiao 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2019-08-22 00:00:00 , DOI: 10.1021/acs.orglett.9b02535 Peng-Zi Wang 1 , Bin-Qing He 1 , Ying Cheng 1 , Jia-Rong Chen 1 , Wen-Jing Xiao 1, 2
Affiliation
|
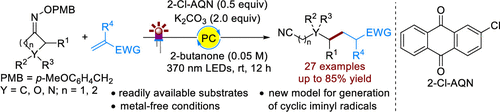
A light-driven, metal-free, and iminyl radical-mediated ring-opening C-C bond cleavage/addition cascade of O-4-methoxybenzyl oxime ethers and alkenes is described for the first time. The reaction shows a broad substrate scope and high functional group compatibility with both components, giving the corresponding valuable oxo nitriles in generally good yields. Key to the success of this protocol is the generation of cyclic iminyl radicals from the O-4-methoxybenzyl oxime ethers via a photocatalytic hydrogen atom transfer (HAT) process. The proposed main pathway is also supported by the preliminary mechanistic studies.
中文翻译:

由光生环亚胺基自由基实现的苄基环酮肟肟的自由基CC键裂解/加成级联。
首次描述了O-4-甲氧基苄基肟醚和烯烃的光驱动,无金属和亚氨基自由基介导的开环CC键裂解/加成级联反应。该反应显示出较宽的底物范围和与两种组分的高官能团相容性,从而以通常良好的收率得到了相应的有价值的羰基腈。该协议成功的关键是通过光催化氢原子转移(HAT)过程从O-4-甲氧基苄基肟醚中生成环状亚氨基。初步的机理研究也为拟议的主要途径提供了支持。
更新日期:2019-08-22
中文翻译:

由光生环亚胺基自由基实现的苄基环酮肟肟的自由基CC键裂解/加成级联。
首次描述了O-4-甲氧基苄基肟醚和烯烃的光驱动,无金属和亚氨基自由基介导的开环CC键裂解/加成级联反应。该反应显示出较宽的底物范围和与两种组分的高官能团相容性,从而以通常良好的收率得到了相应的有价值的羰基腈。该协议成功的关键是通过光催化氢原子转移(HAT)过程从O-4-甲氧基苄基肟醚中生成环状亚氨基。初步的机理研究也为拟议的主要途径提供了支持。






























 京公网安备 11010802027423号
京公网安备 11010802027423号