当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
La[N(SiMe3)2]3-Catalyzed Ester Reductions with Pinacolborane: Scope and Mechanism of Ester Cleavage
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-09-04 , DOI: 10.1021/acscatal.9b02605 Christopher J. Barger 1 , Alessandro Motta 1, 2 , Victoria L. Weidner 1 , Tracy L. Lohr 1 , Tobin J. Marks 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-09-04 , DOI: 10.1021/acscatal.9b02605 Christopher J. Barger 1 , Alessandro Motta 1, 2 , Victoria L. Weidner 1 , Tracy L. Lohr 1 , Tobin J. Marks 1
Affiliation
|
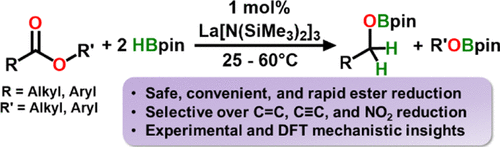
Tris[N,N-bis(trimethylsilyl)amido]lanthanum (LaNTMS) is an efficient, highly active, and selective homogeneous catalyst for ester reduction with pinacolborane (HBpin). Alkyl and aryl esters are cleaved to the corresponding alkoxy- and aryloxy-boronic esters which can then be straightforwardly hydrolyzed to alcohols. Ester reduction is achieved with 1 mol % catalyst loading at 25–60 °C, and most substrates are quantitatively reduced in 1 h. Nitro, halide, and amino functional groups are well tolerated, and ester reduction is completely chemoselective over potentially competing intra- or intermolecular alkene or alkyne hydroboration. Kinetic studies, isotopic labeling, and density functional theory calculations with energetic span analysis argue that ester reduction proceeds through a rate-determining hydride-transfer step that is ligand-centered (hydride is transferred directly from bound HBpin to bound ester) and not through a metal hydride-based intermediate that is often observed in organolanthanide catalysis. The active catalyst is proposed to be a La-hemiacetal, [(Me3Si)2N]2La-OCHR(OR)[HBpin], generated in situ from LaNTMS via hydroboronolysis of a single La–N(SiMe3)2 bond. These results add to the growing compendium of selective oxygenate transformations that LaNTMS is competent to catalyze, further underscoring the value and versatility of homoleptic lanthanide complexes in homogeneous catalytic organic synthesis.
中文翻译:

频哪醇硼烷对La [N(SiMe3)2] 3催化的酯还原反应:酯裂解的范围和机理
Tris [ N,N-双(三甲基甲硅烷基)酰胺基镧(La NTMS)是一种高效的,高活性的,选择性的均相催化剂,用于与频哪醇硼烷(HBpin)还原酯。将烷基和芳基酯裂解成相应的烷氧基-和芳氧基-硼酸酯,然后可以将其直接水解成醇。在25–60°C的条件下,催化剂负载量为1 mol%时,酯的减少量得以实现,大多数底物在1 h内被定量还原。硝基,卤化物和氨基官能团具有良好的耐受性,与潜在竞争的分子内或分子间烯烃或炔烃硼氢化反应相比,酯还原反应具有完全的化学选择性。动力学研究,同位素标记,能量泛化分析的密度泛函理论和密度泛函理论认为,酯的还原是通过以配体为中心的决定速率的氢化物转移步骤进行的(氢化物直接从结合的HBpin转移到结合的酯),而不是通过金属氢化物的中间体通常在有机镧系元素催化中观察到。提出了活性催化剂为半缩醛,[(Me3 Si)2 N] 2 La-OCHR(OR)[HBpin],由La NTMS通过单个La–N(SiMe 3)2键的氢硼解氢反应原位生成。这些结果增加了La NTMS催化的选择性含氧化合物转化的汇编,从而进一步强调了均相镧系元素络合物在均相催化有机合成中的价值和多功能性。
更新日期:2019-09-05
中文翻译:

频哪醇硼烷对La [N(SiMe3)2] 3催化的酯还原反应:酯裂解的范围和机理
Tris [ N,N-双(三甲基甲硅烷基)酰胺基镧(La NTMS)是一种高效的,高活性的,选择性的均相催化剂,用于与频哪醇硼烷(HBpin)还原酯。将烷基和芳基酯裂解成相应的烷氧基-和芳氧基-硼酸酯,然后可以将其直接水解成醇。在25–60°C的条件下,催化剂负载量为1 mol%时,酯的减少量得以实现,大多数底物在1 h内被定量还原。硝基,卤化物和氨基官能团具有良好的耐受性,与潜在竞争的分子内或分子间烯烃或炔烃硼氢化反应相比,酯还原反应具有完全的化学选择性。动力学研究,同位素标记,能量泛化分析的密度泛函理论和密度泛函理论认为,酯的还原是通过以配体为中心的决定速率的氢化物转移步骤进行的(氢化物直接从结合的HBpin转移到结合的酯),而不是通过金属氢化物的中间体通常在有机镧系元素催化中观察到。提出了活性催化剂为半缩醛,[(Me3 Si)2 N] 2 La-OCHR(OR)[HBpin],由La NTMS通过单个La–N(SiMe 3)2键的氢硼解氢反应原位生成。这些结果增加了La NTMS催化的选择性含氧化合物转化的汇编,从而进一步强调了均相镧系元素络合物在均相催化有机合成中的价值和多功能性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号